
Authorities are pushing for CE; manufacturers prefer to focus on value.

Authorities are pushing for CE; manufacturers prefer to focus on value.

The FDA is poised to gain more authority and resources to ensure product quality.

The 45 comments submitted raised concerns about legacy products and ongoing process monitoring.

Broader transparency in product prices and payments to researchers aim to curb conflicts of interest and rationalize drug expenditures.

With cuts in healthcare, biotech drugs are under scrutiny.

FDA aims to regain public confidence in 2009.

QbD can help satisfy FDA and EMEA requirements.

To expand coverage amidst the economic crisis, Obama will be looking hard for ways to cut healthcare costs.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.

The FDA and other regulatory authorities are evaluating new regulations to ensure the safety and quality of nanomaterials in biomedical products.
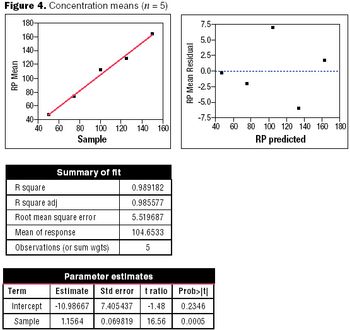
Well-designed experiments can reduce the risk of coming to an incorrect conclusion during a process characterization, assay validation, or process validation study.

The FDA's QbD pilot program is supporting good manufacturing on a global basis.

Every biotech company reaches a point in its development where it must decide what path it will take after it passes the start-up phase. This article discusses what the company must consider to decide what business model it will follow.

The heparin debacle and other crises involving imported drugs and biologics has put pressure on the US FDA to step up its oversight of foreign drug manufacturing.

The Sentinel System aims to generate more adverse event reporting by health professionals, to analyze health information more effectively, and to enhance FDA methods for communicating new safety information to providers and patients.
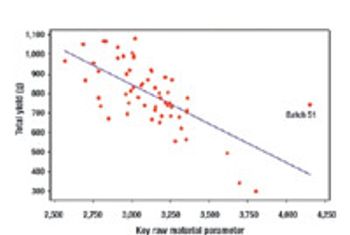
Quality by Design and Design Space can be used by companies to enhance process understanding, improve scientific rigor, and enhanced qualitative and quantative performance, as well as cost savings.

The US Food and Drugs Administration is boosting its efforts for orphan drugs development.

The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.

The FDA is under attack from all sides. Many influential members of Congress either don't trust the agency to monitor the industry appropriately, or have found it politically expedient to keep sounding alarms about inadequate oversight of food and drug safety and clinical research. The good news is that there seems to be a growing consensus that FDA needs a major infusion of cash to regain its stature as an effective science-based regulatory agency.
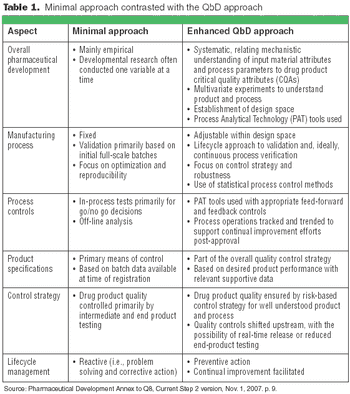
The principles of QbD can be applied to biotech development and manufacturing to help resolve many common issues. QbD scientifically provides a greater understanding of the complex relationships among product quality attributes, the manufacturing process, and clinical safety and efficacy by determining the various permutations of critical input variables that will keep the product within specification.

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

More informed submissions may lead to regulatory flexibility for postapproval changes.
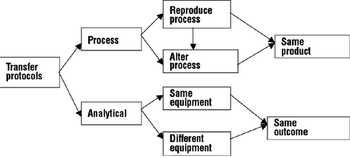
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

The much needed modernization of the agency's IT systems and inspection capabilities will likely fall prey to budget shortfalls.

The new year begins on a note of optimism. A major breakthrough in stem cell research promises to open the door to new biomedical research opportunities. The drawn-out Congressional debate over user-fee reauthorization and drug safety regulation is over, and most parties seem satisfied with resulting compromises. The vaccine industry is experiencing a resurgence after years in the doldrums, with important new vaccines on the market and more under development. And unlike many previous years, the US Food and Drug Administration (FDA) had a confirmed commissioner for all of 2007 and relatively stable leadership.