
An approach to reduce batch time, increase productivity, and decrease costs.

An approach to reduce batch time, increase productivity, and decrease costs.

Top priorities for manufacturers include user fees, new health initiatives, and regulatory compliance.

Changes on Capital Hill create uncertainty for healthcare reform, drug regulation, and biomedical research.

Best practices to strengthen supplier quality management.

Comparative effectiveness poses challenges for drug manufacturers.

A new strategy to streamline vaccine development and oversight.

Too many REMS cause headaches for doctors and the industry.

What small biotechs need to know about quality management systems.

Plant closures, product recalls prompt FDA re-evaluation of GMP enforcement efforts.
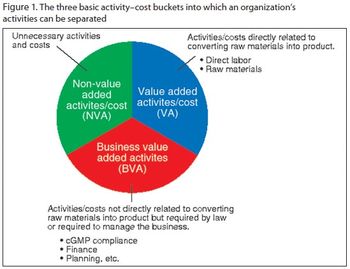
By identifying and eliminating non-value-added activities, drug manufacturers can avoid falling into the same cost-traps in the future.

More information may be available on drug approvals, prices, and research to expand public understanding of regulatory policies.

International outsourcing and rising theft spur regulatory action and manufacturer oversight.

Broader benefits and biosimilars will offset hefty fees and discounts while preserving R&D incentives.

The FDA is expanding postmarketing safety requirements, despite limited resources to manage these added responsibilities.

An effective CAPA plan provides a mechanism for responding to the unexpected.

The new Sentinel system aims to expand access to data on medical product safety and patient effects.

Trouble at Genzyme and with flu vaccine production illustrates the challenges in producing safe and potent biologics.

How will implementing Quality by Design strategies affect your compliance status?

Demand for new vaccines and therapies in 2010 will be offset by concerns about drug prices and product safety.

Vaccine research and development is surging, but continues to face manufacturing and regulatory challenges.

The focus on the design space will lead to a new workspace, and will affect staff in the development, manufacturing, and quality functions.

Heightened attention to product safety issues is slowing the approval process for new therapies.

Stiffer enforcement of quality standards aims to restore public confidence in agency actions.

The FDA is encouraging manufacturers to invest in research and development for new vaccines and therapeutics to combat third-world diseases.

Pressures to reduce healthcare spending generates proposals to spur competition, cut costs.