
FDA publishes guidance on ANDA submissions.

FDA publishes guidance on ANDA submissions.

EMA releases details of restructuring.

FDA updates guidance to reflect advances in technology.

Establishing a well-defined training program is a crucial activity for any biopharmaceutical organization.

Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

European governments are under pressure to take regulatory action, but solving the problem of medicine shortages is not as straightforward as it seems.

Increased manufacturer outsourcing requires clear policies and written agreements with CMOs.

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.

A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

The author presents best practices for extractables and leachables.

Have FDA initiatives improved manufacturing quality?

Latin America's diverse growing market seeks regulatory harmonization.

Opioid abuse generates calls for efforts to curb distribution.

EU authorities are stepping up their efforts to incorporate QbD principles.
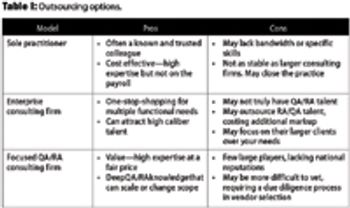
This article examines the options to best match needs and spending for quality and regulatory leadership.

The authors present solutions based on a review of current service offerings and their audit experience.

Vaccine development is benefiting from manufacturing advances.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.

USP's focus in 2013 involves standards relating to organic impurities, measurement of residual DNA and host-cell proteins in biotechnology products, and elemental impurities.

Discussions are underway as the pharmaceutical sector calls for greater consistency in the global monitoring of GMP compliance and quality testing of APIs and finished medicines.

Shortages spur efforts to overhaul manufacturing oversight.

Shortages spur efforts to overhaul manufacturing oversight.