
The company is launching a new pre-fabricated, ready-to-run manufacturing facility that is expected to significantly decrease the production timeline for viral vector-based therapeutics.

The company is launching a new pre-fabricated, ready-to-run manufacturing facility that is expected to significantly decrease the production timeline for viral vector-based therapeutics.

The new facility, to be built in Toronto, Canada, will significantly increase capacity for pediatric and booster vaccines.

The biopharmaceutical firm has chosen Rhode Island as the site of its next-generation biomanufacturing plant, which will offer flexibility, speed, and efficiency.

The new 300,000-square-foot facility is considered the largest dedicated cell and gene therapy manufacturing facility with fully integrated services.
Developments and investments in single-use systems advance upstream biomanufacturing.

The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.

Sartorius has delivered to ABL’s Strasbourg facility, a GMP viral vector manufacturing package solution that includes single-use bioreactors and an automation platform for normal flow filtration, tangential filtration, and mixing.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

Ompi EZ-fill vials and Daikyo Seiko PLASCAP press-fit closures are a confirmed product set for use with Vanrx Pharmasystems' Aseptic Filling Workcells.

Amgen plans to invest approximately $300 million in a new biomanufacturing plant in the United States.

The Flexicon PF7 peristaltic tabletop aseptic liquid-filling machine from Watson-Marlow Fluid Technology Group is suitable for GMP-regulated biotechnology and pharmaceutical operations.
Single-use technologies are starting to gain ground as capacity needs change, but industrywide adoption remains low.

The new Acquity Arc Bio System by Waters is specifically engineered to enable efficient transfer and improvement of bioseparation analytical methods.

Corning will feature its 3D bioprinting technology and tools to support 3D cell culture at booth #1029 during the SLAS 2018 conference on Feb. 3-7, 2018 in San Diego, CA.

Comecar will provide cell culture equipment for biopharmaceutical company CO.DON AG's new production site in Leipzig, Germany.

Lonza Pharma & Biotech's's Modular Automated Sampling Technology (MAST) platform, which allows for the collection of up to 10 sterile sample sources for automated analysis in multiple analytical devices, won the CPhI Pharma Award 2017 for Excellence in Pharma: Bioprocessing.

GE Healthcare will equip Cellular Biomedicine Group's cell therapy manufacturing facility in Shanghai, China, with its FlexFactory single-use platform, designed to speed up cell therapy manufacturing timelines.

Sartorius Stedim Biotech will equip Abzena’s contract development and manufacturing facilities in Bristol, PA, and San Diego, CA, with single-use equipment and scale-up technologies.

Proper selection and installation optimizes fluid system performance.

The Wilden Quattroflow QF10k size pump from PSG, a Dover company, has been designed to fill the gap between the existing QF4400/5050 and QF20k pump sizes.
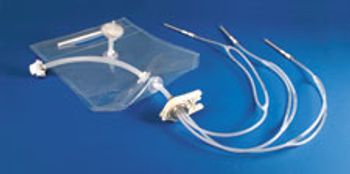
Sterile-molded filling assemblies from AdvantaPure are suited for single-use vial and syringe filling.
Recent investments show expansion activity in cell culture facilities.

MilliporeSigma will collaborate with IPS and G-CON to offer end-to-end, turnkey, modular MAb manufacturing.

SciLog Select Go Single-Use Assemblies from Parker Hannifin uses an assortment of validated parts and assemblies to build needed devices for biopharmaceutical manufacturing.