
Single-use systems may offer a suitably flexible solution to the biomanufacturing challenges of cost and time-to-market.

Single-use systems may offer a suitably flexible solution to the biomanufacturing challenges of cost and time-to-market.

This article provides a review of various 2000-L single-use bioreactors used by biopharmaceutical suppliers for commercial production.

The authors present a risk analysis of the impact of various business and operating risks on three facility layout strategies.

The expansion will increase the company’s single-use capacity to meet growing demand.

Vibalogics has increased its single-use bioreactor and purification capacity with a new manufacturing for its specialist oncolytic virus and viral vector manufacturing services as a result of a growth in demand.
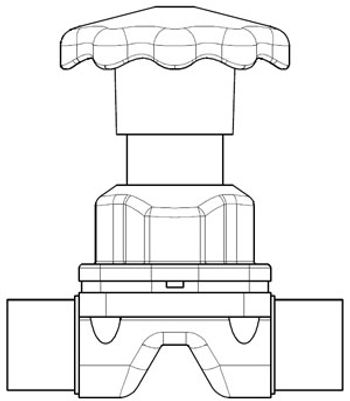
Valve design and materials affect performance and cost to maintain.

Recent upstream processing innovations include enhanced sensor technology, single-use bioreactors, and automated cell culture systems.

Single-use technology is gaining ground in downstream bioprocessing, but challenges stall further adoption.

This article takes a look at current practices for cleaning and sterilizing biomanufacturing equipment used in a multi-product versus single-product setting.

The company’s Quantum peristaltic pump uses a patented single-use cartridge technology and is applicable to downstream bioprocessing.

The BioContinuum Buffer Delivery Platform offers streamlined buffer management and delivers “contiGuous” bioprocessing.

ITT Engineered Valves presented its new EnviZion hygienic valve technology at INTERPHEX 2019 in New York City.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

3D printing offers a new design freedom for bio/pharmaceutical manufacturing: whether for “printing” a solid-dosage drug or for creating a piece of equipment for bio/pharmaceutical laboratories or manufacturing facilities.

Distek, a provider of laboratory testing instruments, added a dual impeller single-use bioreactor (SUB) system to its BIOne portfolio.

A continuous peptide manufacturing process invented by Swedish Biomimetics 3000 Ltd is a platform technology also applicable to other solid-phase chemical processes, such as chromatography.

The acquisition of the site in Copenhagen, Denmark, will significantly expand Fujifilm’s capacity and capabilities.
A look at recent investments by contract manufacturers to increase single-use bioreactor capacity.

Downstream process equipment for mAbs manufacturing must be designed to fit technology developments in upstream processes.

As automation in biomanufacturing becomes more important, so does the need to integrate process data.

Suppliers address the complexity of supplying disposable components for single-use systems to the global biopharmaceutical manufacturing industry.

Single-use components for biopharmaceutical manufacturing have a lower environmental impact than reusable components, but disposal is still a consideration.

Parker Bioscience Filtration added a cleanroom at its site in Birtley, UK for manufacturing single-use assemblies and sensors used in biopharmaceutical processing.

G-CON will provide a complete cleanroom infrastructure for GE Healthcare’s cell therapy and viral vector production platforms that will simplify early-stage manufacturing efforts.

The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.