
The STERIS ProKlenz ONE alkaline cleaner now has a label performance claim for biofilm removal, in addition to the product’s existing label as a disinfectant and virucide.

The STERIS ProKlenz ONE alkaline cleaner now has a label performance claim for biofilm removal, in addition to the product’s existing label as a disinfectant and virucide.

Kimberly-Clark Professional added the Kimtech A5 Sterile Integrated Hood and Mask XL for head shapes, sizes, and hairstyles that pose a challenge to standard aseptic gowning for cleanroom operators.

A collaboration using Pall’s bioprocessing technology in G-CON’s PODs enables flexible continuous bioprocessing and viral vector facility solutions.

The contract development and manufacturing organization expanded its analytical chemistry suite and added a new office in Boston, MA.

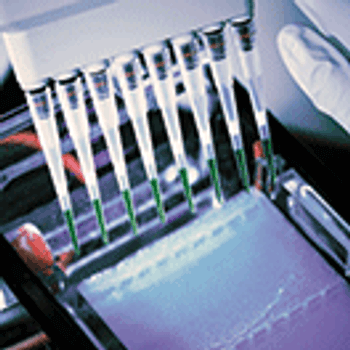
Lonza’s new PyroTec Pro Robotic Solution provides a fully automated workflow for endotoxin detection.
This study was successful in establishing a reliable and effective method for evaluating cleaning processes based on risk. Click here to view a PDF of this article.

Ajinomoto Althea’s Optima VFVM 7000 aseptic fill/finish line supports a range of drug substance APIs.

Revised versions of ISO 14644 Parts 1 and 2 introduce changes to sampling procedures and monitoring plans for cleanrooms and clean zones.

Material compatibility, material sourcing, facility layout, and training are crucial aspects of a successful disposable fill-finish system.

This article discusses cleaning validation of equipment dedicated to the production of a single API.

Meritech’s CleanTech 2000SCA Automated Handwashing System delivers a 12-second wash and rinse cycle that removes 99.98% of dangerous pathogens from bare skin and gloves.


Aseptic Valve Range Increases Sterility

Walker Barrier Systems builds eight mobile clean rooms for the Texas A&M Center for Innovation in Advanced Development and Manufacturing.

To assess current trends in cleanrooms and engineering & facilities, BioPharm International turned to Parrish Galliher, founder and chief technology officer, Xcellerex, Inc.; Jim Maslowski, owner, PDC Aseptic Filling Systems; Morgan Polen, vice president, application technology, Lighthouse Worldwide Solutions; and Benoît Verjans, commercial director, Aseptic Technologies.


A symposium at the AAPS National Biotechnology Conference in Boston, June 19-22, 2006, addressed key concerns and new developments in manufacturing biologic products in a sterile environment.

It is not likely that you will ever personally design and build a cleanroom; however, you may be responsible for the care and upkeep of many.