
Time and sensitivity are essential for analytical technologies in all phases of biopharma development.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.
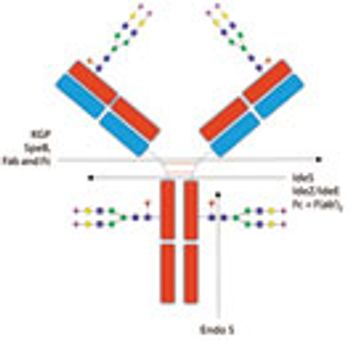
Advances in glycan analysis are enhancing biologics development and quality control processes.
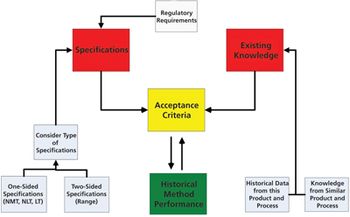
The authors present the results of a survey of biologics manufacturers to evaluate how these manufacturers transfer analytical methods.

Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.
Light scattering analysis combined with more rapid size exclusion chromatography improves protein characterization.

SEC-MALS Detector for UHPLC

The ?DAWN can be attached to any UHPLC system to determine molecular weights and sizes of proteins.

UPLC Separation Intergrates into Mass Spectrometers
USP evaluates raw materials used in the chemical synthesis of peptides.
Liquid Chromatography Column Separates Glycans
USP evaluates quality attributes for synthetic peptides.
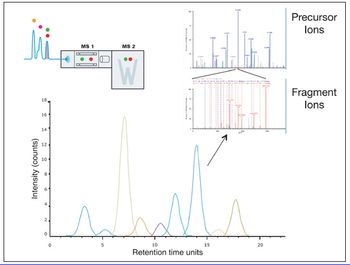
By providing information on the relative accessibility of locations within a protein, HDX by mass spectrometry opens new windows into the higher order structure of biomolecules.