
PharmaResearch’s DOT-based nanoparticle platform enters US clinical testing, highlighting delivery innovation aimed at improving tolerability in solid tumor therapies.

PharmaResearch’s DOT-based nanoparticle platform enters US clinical testing, highlighting delivery innovation aimed at improving tolerability in solid tumor therapies.

NeoVac first-in-human data suggest that optimized lipid nanoparticles may improve mRNA tolerability, enabling repeat dosing and broader therapeutic use.

Integrated CDMO networks streamline complex drug development across advanced modalities like ADCs.

Study data show high-dose nusinersen can improve function and slow neurodegeneration, informing future SMA dosing strategies and lifecycle management.

The Eisai–Henlius partnership expands Japan’s access to a differentiated PD-1 antibody, highlighting cross-border oncology deals targeting high unmet need.

AI, biomarkers, and novel oncology modalities are driving evolution in biopharma pipelines, according to Nmblr CEO Janice MacLennan.

A new Cellares–Stanford collaboration aims to demonstrate how automated platforms could standardize gene-edited stem cell manufacturing and accelerate clinical translation.

FDA’s acceptance of Affinia Therapeutics’ IND positions the company to test lower-dose, heart-targeted AAV gene therapy for BAG3 cardiomyopathy.

The SK bioscience–Gates MRI agreement highlights how scalable mAbs could broaden infant RSV prevention beyond high-income markets.

Industry experts discuss the standout packaging trends from 2025 that will influence the industry’s future.
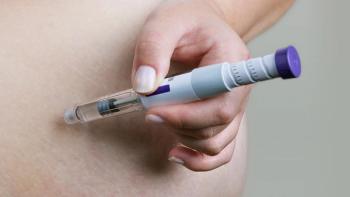
The VESPER-3 trial results show that extended-interval GLP-1 dosing may expand obesity care by balancing sustained weight loss with reduced injection burden.

Janice MacLennan, CEO of Nmblr, notes how AI is transforming biopharma R&D and market access, forcing teams to prove value beyond science and redesign work around human strengths.

AdvanCell and 48Hour Discovery align peptide discovery with a Lead-212 infrastructure to build a durable targeted alpha therapy pipeline aimed at oncology markets.
This new licensing deal with SanegeneBio signals growing confidence in RNAi technologies.

The facility builds on Eli Lilly's $50 Billion investment strategy to strengthen the company's domestic manufacturing capabilities.

Agentic AI enables closed-loop, autonomous drug discovery workflows that accelerate biopharma R&D, improve efficiency, and reshape innovation.

Such targeted private investments underscore the growing importance of early manufacturing scale-up in de-risking immunomodulatory biologics for neurodegenerative diseases.

The company’s major long-term capital commitment highlights how global drugmakers are anchoring future innovation, advanced modalities, and supply resilience within China’s life sciences ecosystem.

The guidance provides clarity to drug manufacturers on offering lower drug prices directly to patients, including patients on Medicare and Medicaid.
Cellares’ $257M Series D signals growing industry urgency to industrialize cell therapy manufacturing through automation and scalable production models.

Regulatory review of Eisai’s subcutaneous lecanemab highlights how delivery innovation may expand access and scalability for disease-modifying Alzheimer’s therapies.

This fast track designation signals growing regulatory momentum for trispecific antibodies as the myeloma field pushes beyond single-target immunotherapies.

Integrated biotech–CDMO partnerships are becoming critical as fusion protein complexity pushes developers to align discovery and manufacturing earlier.

Survey results show a shift in pharma industry sentiment from optimism to caution as rising insecurity offsets high satisfaction and intellectual engagement.

The European Commission’s approval of GSK’s Arexvy for adults 18 years and older expands the respiratory syncytial virus vaccine market and signals broader adoption of adult immunization strategies.

Green chemistry is emerging as a strategic lever for biopharma manufacturing, enabling safer processes, efficiency gains, and long-term competitiveness.

Biopharma manufacturing faces a global workforce crisis, driving adoption of continuous upstream automation to protect and scale capacity, says Eric Langer of BioPlan Associates, in the continuation of his industry outlook.

The decision is based on President Trump’s executive order issued last year.

Mendra’s launch highlights the growing use of AI to improve patient identification, clinical execution, and commercialization in rare disease programs.

Next-gen antibody R&D is shifting to multifunctional modalities driven by manufacturing scale and regulatory acceleration.