
Toni Manzano, PhD, discusses his CPHI Europe presentation, stating that AI is essential for managing complex drug and biologics manufacturing.

Toni Manzano, PhD, discusses his CPHI Europe presentation, stating that AI is essential for managing complex drug and biologics manufacturing.

Biopharma innovators were recognized at CPHI 2025 for advances in drug delivery, continuous bioprocessing, and supply chain capabilities shaping global therapies.

Toni Manzano, PhD, Azion, says AI integration in drug manufacturing necessitates regulatory risk assessment, cloud infrastructure for big data, and strict data integrity compliance.


According to a CPHI Frankfurt keynote panel, Europe's biopharma sector must accelerate adaptation to new regulations to stay competitive with the US and China.

Toni Manzano, PhD, Aizon, says AI adoption in biopharma, supported by new GXP guidelines, is challenged by poor data quality and the complexity of industrializing many specific models.

Silvia Scaglione of React4Life explores how organ-on-chip systems, AI, and multi-organ models are revolutionizing preclinical testing and advancing personalized therapeutics.

In the first of two parts, Viktoria Enkmann addresses problems with quality control for personalized medicines in the context of lipid nanoparticles and RNA therapeutics overall.

Enzyme engineering holds promise, but Marina Cañellas of Zymvol Biomodeling says the enzymes themselves need to be improved through increased discovery and investigation to be able to achieve a greater amount of chemical reactions.

Vishal Mukund Sonje, Vaccine Manufacturing Lead, CEPI, talks about the challenges that arise in the manufacturing of vaccines in various global regions and gives a preview of his presentation at CPHI Frankfurt 2025.

Organ-on-chip platforms and AI integration are revolutionizing drug development, personalizing medicine, and advancing pharma research, says React4Life’s Silvia Scaglione.

Novartis’ acquisition accelerates RNA drug innovation, expanding genetic therapy pipelines and reshaping biomanufacturing strategies for neuromuscular disease.

Pharmaceutical Technology® spoke with Tara Dougal, Event Director for Pharma at Informa Markets about what attendees at CPHI Frankfurt should expect this year.

News from the week emphasizes improvement of patient access and supply-chain resilience through AI-driven manufacturing and digital traceability.
Abzena’s antibody platform enables Orion to streamline biologics development, showcasing trends in scalable, risk-reduced manufacturing for the industry.

SK pharmteco expands US peptide production capacity, strengthening domestic API manufacturing and accelerating biopharma process development.

In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates, and Siegfried Schmitt, Parexel, give their opinions on why those working in the pharmaceutical industry should lend their voices to draft regulations.

The partnership is aiming to embed digital identification into labware, solving chain-of-identity challenges for individualized cell and gene therapies.

Non-parenteral alternatives for biologics remain a clinical imperative and a formidable challenge.

In episode 27, Christopher Hopkins, PhD; Omkar Kawalekar, PhD; Barnaby Pickering; and Jerry Rosenbaum, MD, go behind the headlines.

Fluorescence spectroscopy with A-TEEM offers rapid, precise monitoring of cell culture media variability for improved biopharmaceutical quality control.

CHMP’s positive opinion for a subcutaneous form of anifrolumab paves the way for a once-weekly at-home lupus treatment option in Europe.

A new leadership summit and expert tracks explore AI, sustainability, and next-gen manufacturing shaping the future of global biopharmaceutical development.
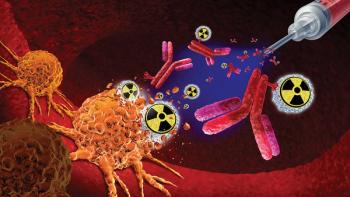
Michael Ritchie, chief commercial officer at Champions Oncology, explains what makes radiopharmaceuticals unique in the treatment of cancer.
This quiz measures your comprehension of one of our recent feature articles.

This quiz measures your comprehension of one of our recent feature articles.

The BioPharm International biopharma industry report on digital transformation uncovers an adoption paradox. This video summarizes our findings.

This week’s news highlights biopharma R&D’s focus on new biologics and CGT innovation, with growth fueled by AI adoption and significant investments into market expansion.

ARM board member Miguel Forte highlights education programs, innovation hubs, and hands-on training to foster workforce expertise in the biopharma industry.