
The European Commission’s new structure has sparked controversy about its allocation of responsibilities and the impact on the development and approval of new medicines.

The European Commission’s new structure has sparked controversy about its allocation of responsibilities and the impact on the development and approval of new medicines.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.

Switching grades of raw material late in the development cycle can be costly. Best practice says get it right at the beginning.
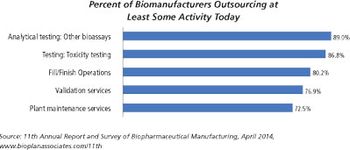
There are significant differences between small molecules and biologics fill/finish capacity.

Manufacturers are under pressure to develop pipelines, promote quality, and justify pricing.

In late 2014, standards organizations continued to work towards harmonization and securing drug safety.
The author presents opportunities and challenges in implementing the product lifecycle approach.
Ligand-binding assays are fundamental to characterizing biosimilars.

New designations lead to faster drug approvals, but there is more work to be done.

As more biologic drugs come to market, manufacturers will require improved bioprocessing technologies.

The authors review efforts to limit polymer degradation without significantly impeding cell growth.





Click the title above to open the BioPharm International January 2015 issue in an interactive PDF format.