
Swissmedic grants conditional marketing authorization for Novavax COVID-19 vaccine for individuals aged 18 years or older in Switzerland.

Swissmedic grants conditional marketing authorization for Novavax COVID-19 vaccine for individuals aged 18 years or older in Switzerland.

FDA is reviving efforts to establish a Quality Management Maturity program.

Orchard Therapeutics announces reimbursement agreement, which will make Libmeldy available for all eligible MLD patients in Italy.

Dupixent has been approved by the EC for children aged 6 to 11 years with severe asthma with type 2 inflammation.

Democrats have proposed a measure to facilitate access to less costly comparator drugs needed in clinical trials, while another bill aims to increase transparency in the costs of clinical trials.

FDA has granted Priority Review to Roche’s Actemra/RoActemra for the treatment of COVID-19 in hospitalized adults.

ECDC and EMA have concluded that it is too early to consider using a fourth dose of mRNA COVID-19 vaccines in the general population.

Teva has initiated a voluntary nationwide recall of one lot of IDArubicin Hydrochloride Injection USP 5mg/5 mL due to the presence of particulate matter.

UK’s MHRA has issued a positive opinion for the Early Access to Medicines Scheme for radioligand therapy in patients with advanced prostate cancer.

FDA has accepted Dupixent for Priority Review in patients aged 12 years and older with eosinophilic esophagitis.

ATMPS Ltd has been granted a patent from the United States Patent and Trademark Office for the use of its Hataali blockchain technology used in personalized medicines.

FDA has granted an expanded Emergency Use Authorization to Pfizer and BioNTech’s COVID-19 vaccine boosters in individuals aged 50 years and older.

Legislative proposals set the stage for debate on FDA policies and programs and whether to include some measures in legislation to reauthorize user fees.

Data may be used to improve (or remove) a corrective action/preventive action.
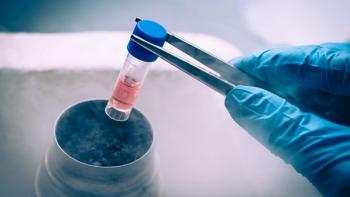
Taking key considerations into account along the cell therapy supply chain can set cell therapy developers up for success.

Performing corrective action and preventive action (CAPA) activities is often necessary to investigate a manufacturing deviation and prevent it from reoccurring. BioPharm International asked Joe O’Gorman, head of Global Operations at LZ Lifescience, a Cognizant Company, about the role technology plays in performing CAPAs.

Quality metrics and more domestic production aim to avoid supply disruptions and drug shortages.

Experts Susan J. Schniepp, distinguished fellow for Regulatory Compliance Associates, and Steven J. Lynn, executive vice-president, Pharmaceuticals for Regulatory Compliance Associates, discuss the verification of compendial methods.

Consumer advocates continue to press for initiatives to lower drug prices by revising or reducing patent protections.

Pfizer has issued a voluntary nationwide recall on lots of Accuretic tablets due to high levels of nitrosamine.

Congress is considering revising and improving policies related to drug development and regulation for possible inclusion in broader legislation to reauthorize FDA user fees.

Moderna, Pfizer, and BioNTech are seeking FDA approval for a fourth dose of their respective COVID-19 vaccines.

Testing cleanroom garments while in use and during daily cleanroom operations is vital for contamination control.

Why critical thinking must be applied before technology to ensure regulatory compliance.

Experts weigh in on the current state of computer validation and its role in the greater regulatory landscape.