The development and optimization of an efficient conjugation process involves identifying the critical quality attributes and monitoring critical process parameters.

The development and optimization of an efficient conjugation process involves identifying the critical quality attributes and monitoring critical process parameters.
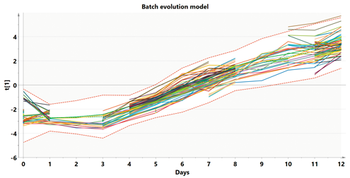
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.
As closure integrity testing moves from a probabilistic to a deterministic basis, designs are promoting improved control and reduced operator contact.

A quality-by-design approach that implements PAT offers advantages in upstream cell-culture processing.
Cross-functional reliability rooms identify risk and planning metrics, provide insights for production forecasts, and predict trends and areas for improvement.

The partnership and the formation of the institute intend to bring together industry, academia, and regulators to tackle challenges and provide solutions for continuous manufacturing.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
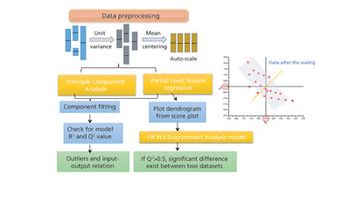
The authors review major developments in use of MVDA in bioprocessing applications.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.
A quality-by-design approach that defines potential viral contaminants of source materials can be used to achieve viral clearance.

Experts give insight on method transfer, QbD, and regulations for analytical method development and validation for biopharmaceuticals.

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.

Agencies extend successful pilot program to further harmonization of QbD topics.

Wolfgang Weikmann of Vetter Pharma discusses the implementation of quality by design in sterile manufacturing.

EMA and FDA publish joint QbD guidance on design space verification.

As the complex requirements of manufacturing biologics are manifold, it is important that biomanufacturing companies adopt quality-by-design principles.

Agencies collaborate to ensure consistent product quality.

Industry experts discuss the implementation of QbD and PAT tools in biopharmaceutical manufacturing.
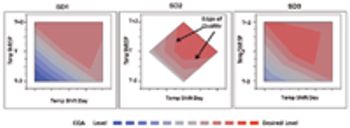
Traditional project decision-making vs. a QbD approach.

EU authorities are stepping up their efforts to incorporate QbD principles.

Understanding overall supplier capability versus the critical-to-quality attributes of your product can reduce both risk and cost.
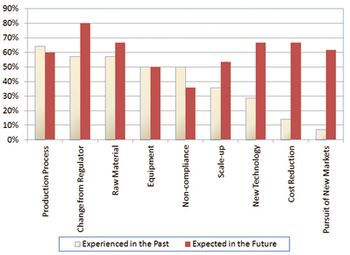
MIT survey results address product and site characteristics that statistically correlate with quality performance.

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.
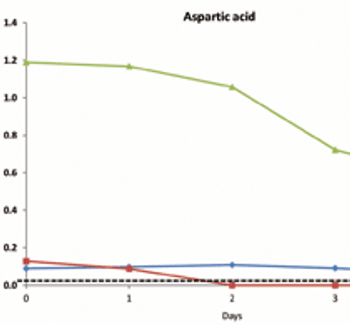
Current expectations in bioprocessing and a framework for using NMR to enhance a QbD approach.
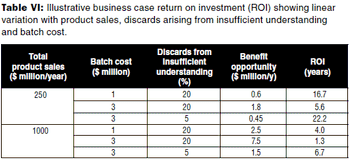
The author describes a methodology for developing a per product qualitative and semi-qualitative business case for applying QbD to a biopharmaceutical product.