
Global supply chain experts examine implications of the DSCSA as well as topical considerations around the EU's FMD in this serialization video series ft. manufacturing, packaging, software, and regulatory experts.

Global supply chain experts examine implications of the DSCSA as well as topical considerations around the EU's FMD in this serialization video series ft. manufacturing, packaging, software, and regulatory experts.

Global supply chain experts examine implications of the DSCSA as well as topical considerations around the EU's FMD in this serialization video series ft. manufacturing, packaging, software, and regulatory experts.

The biopharma industry is seeing more merit in strategizing clinical and commercial drug development as early as the preclinical phase.

Global CDMO, ten23 health, has acquired filling technology and drug product manufacturing expert, swissfillon, enhancing its integrated offering.

Spectrum Chemical scientists give a shout-out to 10 chemicals impacting quality of life in conjunction with National Chemistry Week.

MilliporeSigma announced the opening of its second Carlsbad, California-based facility, which doubles production capacity to support commercial and industrial manufacturing of viral vectors.

For some manufacturers, the goal was to be fully compliant with the DSCSA, including both serialization and aggregation, from day one.

Pharmalex has launched the Biopharma Excellence brand, a new service line that combines the expertise of PharmaLex, ERA Consulting, and Biopharma Excellence.

SGS is investing in a new E&L lab in Navi Mumbai, India, that will provide testing services.

Avid Bioservices is building a viral vector development and manufacturing facility near its existing biologics manufacturing facility in California.

Speed-to-market, capacity, technology development, and increased investment top the list.
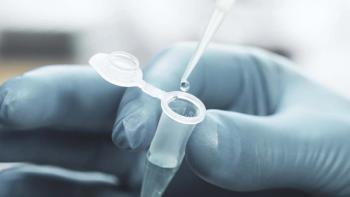
Establishing bioassay studies for biosimilar development is important for supporting regulatory filings.

AGC Biologics is expanding manufacturing capacity at its Heidelberg, Germany, facility for plasmid DNA and messenger RNA.

Febles will oversee day-to-day aseptic manufacturing at Pharmaceutics International, Inc.

As interest in cell and gene therapies continues to grow, the need for safe and consistent reagents to support research, development, and manufacture efforts also increases.

BioPharm International asked Vincent Colicchio, vice president, supply chain and external manufacturing at Dr. Reddy’s Laboratories, about how a lack of proper supplier oversight can impact the pharmaceutical industry and the drug supply chain.

Quotient Sciences is investing £6.3 million ($8.68 million) into their drug substance manufacturing capabilities.

Almost half of pharmaceutical industry profits continue to come from biopharmaceuticals.

AGC Biologics has broken ground on a new multipurpose facility in Copenhagen, Denmark, that will increase capacity.

Lonza plans to establish drug product manufacturing capabilities at is site in Guangzhou, China, to produce clinical trial and commercial supply in the country.

Biotage’s new facility in Cardiff, UK, will produce lipids using large-scale flash purification.

The CRO market will see greater consolidation by both financial buyers looking to consolidate smaller players and larger CRO players wanting to expand into value-added specialty service areas.

Scenario modeling informs planning for current and future manufacturing needs.

Manufacturers should consider the benefits and risks of contract packaging for stability studies.

LGM Pharma will offer contract analytical testing and stability services to pharmaceutical developers and manufacturers.