
CordenPharma’s new starter kits are designed to enable effective formulation in the development of mRNA-based therapeutics.

CordenPharma’s new starter kits are designed to enable effective formulation in the development of mRNA-based therapeutics.

CSL will share access to Cytegrity, its proprietary stable production system for lentiviral vector production, with Genezen.

Daiichi Sankyo is investing approximately €1 billiion (US$1.08 billion) to expand its Pfaffenhofen an der Ilm, Germany, site for ADC development and production.

AstraZeneca has completed its acquisition of Icosavax, gaining the IVX-A12 vaccine candidate that targets RSV and hMPV.

Webinar Date/Time: Wed, Mar 20, 2024 11:00 AM EDT

Challenges in fermentation can be addressed through equipment changes, facility design, and process development.

FDA has approved Iovance Biotherapeutics’ Amtagvi (lifileucel) for treating patients with unresectable or metastatic melanoma.

Webinar Date/Time: Tue, Feb 13, 2024 11:00 AM EST

Under the new partnership, Samsung Biologics will develop and manufacture an antibody for a LegoChem Biosciences ADC candidate.

Webinar Date/Time: Tue, Mar 5, 2024 2:00 PM EST

Purolite and Repligen have commercially launched a new CH1 affinity resin for the purification of specialized mAbs.

Novo Holdings has entered into a merger agreement with Catalent and will acquire Catalent in a deal valued at $16.5 billion.

Under a new collaboration, Lonza and Oxford Nanopore aim to commercialize a CGMP-validated test for advanced analysis of mRNA products.
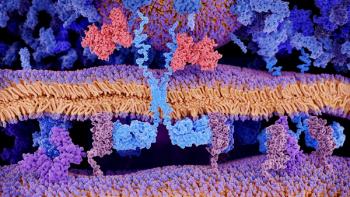
Despite slow growth over recent years, the CAR-T cell therapy space is expected to see considerable advancements in the near future.
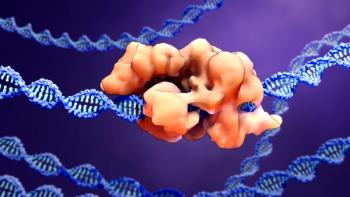
The milestone approval of a gene-edited therapeutic paves the way for gene-editing technologies.

SUS aids biopharma manufacturers to overcome the rigidity of more traditional stainless-steel technologies.
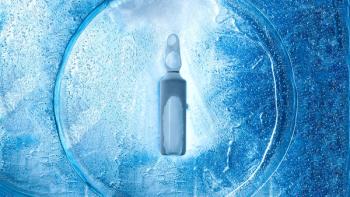
Developing freeze-drying processes requires patience and deep product and process understanding.

The final guidance provides specific recommendations for CMC, pharmacology, toxicology, and clinical study design for CAR-T cell products.

Given its positioning at each new year’s commencement, the J.P. Morgan Healthcare conference helps establish the tone with which the pharmaceutical industry is viewed.

Webinar Date/Time: Thu, Feb 22, 2024 11:00 AM EST

The 2024 Pharmapack Europe Award winners include companies involved in ground-breaking innovations in novel drug delivery solutions, reusable connected devices, and recyclable packaging.

Under a new alliance, KBI Biopharma and Argonaut Manufacturing Services will combine their strengths to offer end-to-end biopharma development and CGMP manufacturing solutions.

Abzena has launched its AbZelect platforms, designed for improving cell line development.

Charles River’s off-the-shelf rep/cap plasmids are intended to simplify gene therapy supply chains.

Boehringer Ingelheim is investing €120 million (US$131 million) into its Koropi, Greece site to expand production for new therapeutics.