The authors present a review of the techniques commonly used for glycosylation analysis.

The authors present a review of the techniques commonly used for glycosylation analysis.

Probiodrug has signed an agreement with Rentschler Biotechnologie GmbH for the development of PBD-C06, a pGlu-Abeta-specific monoclonal antibody, as treatment for patients with Alzheimer’s disease.

Rafe Swan/Getty Images; Dan WardIrreproducible preclinical research is a global, expensive, and well-recognized problem that contributes to delays a

Aragen Bioscience has licensed ProteoNic Biotechnology’s 2G UNic recombinant protein production technology, which increases manufacturing efficiency and reduces cost of goods for recombinant biologicals.
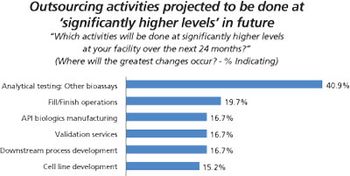
Biopharma companies on both sides of the Atlantic ship more of their assay testing to outside service providers.
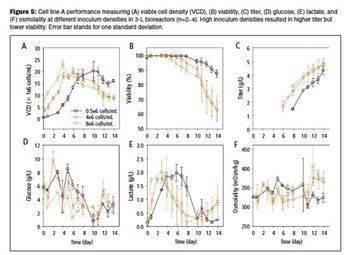
The use of commercially available media to achieve high titer in early process development is discussed.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

Gallus BioPharmaceuticals enters a cell line optimization and manufacturing agreement with Omni Bio Pharmaceutical.

CMC Biologics and OnoSynergy form an agreement from cell-line development.

BioWa and Lonza have entered into a licensing agreement with MedImmune for cell line technology.
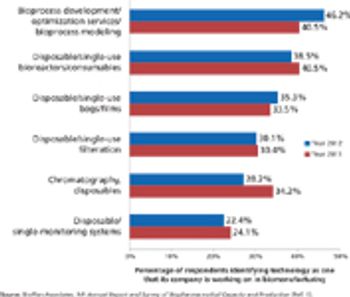
Industry wants more innovation, but can suppliers meet customers' needs?
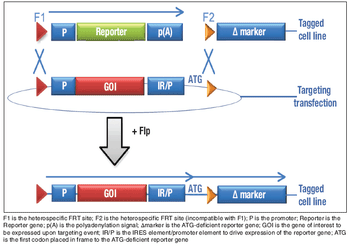
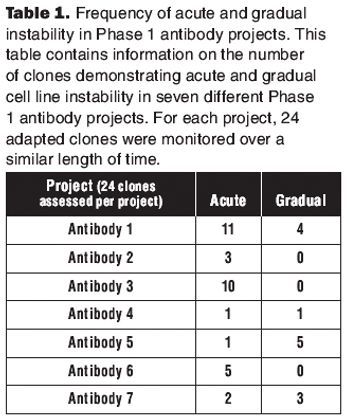
By considering stability as part of the cell line selection and cell banking paradigm, we can ensure that instability problems are not observed during clinical or commercial manufacturing.