
The company is increasing its cell culture media production capacity at its facilities in Pasching, Austria, and Logan, Utah.
A collaboration integrates Sartorius Stedim Biotech’s BIOSTAT STR bioreactors and Repligen’s XCell ATF cell-retention control technology to create simplified, scalable equipment for intensified cell culture.

The company is increasing its cell culture media production capacity at its facilities in Pasching, Austria, and Logan, Utah.

The new platform is expected to speed up cell line development.

Fujifilm acquires cell culture media companies Irvine Scientific Sales Company and IS Japan.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.
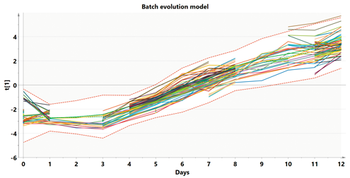
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

The study suggests that circumventing evolution in cell factories can enable the commercialization of new biobased chemicals to large-scale.

Corning will feature its 3D bioprinting technology and tools to support 3D cell culture at booth #1029 during the SLAS 2018 conference on Feb. 3-7, 2018 in San Diego, CA.

Comecar will provide cell culture equipment for biopharmaceutical company CO.DON AG's new production site in Leipzig, Germany.
Recent investments show expansion activity in cell culture facilities.

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

Gilead Sciences will acquire Cell Design Labs to further cell-therapy research and development efforts.

CDMO Celonic acquired a biomanufacturing facility in Heidelberg, Germany from Glycotope.
Anna Kireieva/Shutterstock.comSpeed to market is essential in the biopharmaceutical industry today.

ABEC, an equipment and engineering company, will provide a custom-made, single-use bioreactor to custom manufacturing firm, Emergent, for its Maryland manufacturing facility.

The companies have entered into a strategic collaboration to establish a new cell therapy and regenerative medicine manufacturing platform, which includes a new manufacturing facility in Belgium.

Under an agreement, ProBioGen will develop a stable cell-line for and manufacture an anti-cancer antibody for Chiome using its proprietary cancer cell-killing technology.
Detecting viral contaminants in biologic-based medicines-and identifying their source-requires a holistic testing approach.
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.
Consider automation early in the rollout of clinical translation and scale up of clinical-trial protocols.

FDA noted in a recent inspection that US Stem Cell Clinic was processing and administering stem cell treatments that were neither reviewed nor approved by the agency.

Pfizer will invest $100 million to expand its manufacturing facilities in Sanford, North Carolina.

Media manufacturers are focused on reducing risk, improving quality and consistency, and managing costs.

Perfusion processes can attractive for biologics drug manufacturing; however, obstacles remain.
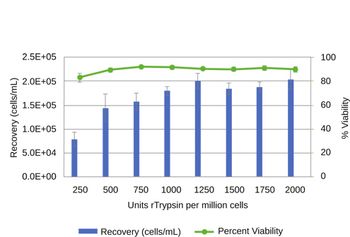
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.

Sartorius Stedim Biotech combined the company’s ambr 15 bioreactor system with the Nova BioProfile FLEX2 cell culture analyzer for laboratory experiments.