
Neil Lewis, chief technology officer at Malvern Instruments, talks about the challenges associated with biologics.

Neil Lewis, chief technology officer at Malvern Instruments, talks about the challenges associated with biologics.

Can postapproval FDA filings immunize pharma companies from patent lawsuits?

AET BioTech and BioXpress Therapeutics have entered into an agreement to codevelop a biosimilar version of Abbott's tumor necrosis factor inhibitor monoclonal antibody adalimumab.
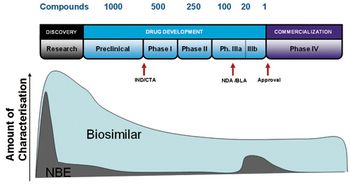
Understanding opportunities and challenges across all major phases of development.

Howard Levine of BioProcess Technology Consultants talks about what industry needs to know to enter the biosimilars game in the US.

Dr. Reddy's Laboratories and Merck Serono, a division of Merck KGaA, have partnered to codevelop a portfolio of biosimilar compounds in oncology.

Industry experts discuss significant achievements. Plus: What's in store for the future.
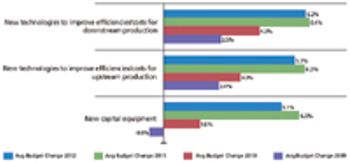
New outsourcing industry sectors have become multibillion-dollar industries.

Does global development have to entail multiple comparability studies?

Key business considerations when developing biosimilar products virtually.

How does working in a virtual biotech environment affect biosimilar development? Anjan Selz outlines target and partner selection in this first of a two part article.

Virtual biotech CEO Anjan Selz focuses on product development, credibility and differentiation.
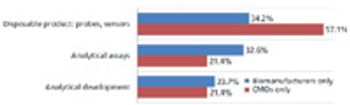
Biosimilar manufacturers need better expression systems and analytical tools to compete.
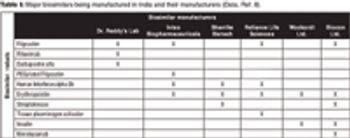
The author explains the current status of India, the challenges, and recommendations that may alleviate these challenges.
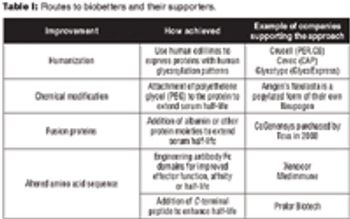
Despite their difficult approval pathway, biobetters offer the potential for innovation and decreased healthcare costs.
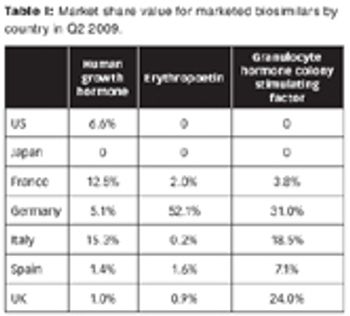
The market landscape for biosimilars is in flux, with limited penetration now, but with the potential for growth for those who can navigate the market. Plus: A SWOT analysis of biosimilars by Anjan Selz.

FDA weighs multiple views regarding the Biologics Price Competition and Innovation Act.

Are biosimilars the next big thing or just the next big bubble?

Follow-on versions of complex biologics require extensive expertise.

So often a leader on the world stage, the US is a laggard when it comes to regulating follow-on biologics.

The third holy grail of biosimilars: interchangeability.

A single standard should apply to all comparability exercises for biologics, be they for biosimilars or manufacturing changes.

The new US legislation will forever alter the commercial landscape for biologics.

New R&D models must be tried, but it will take time to see if they work. In the meantime, a new kind of threat is on the horizon: biobetters.

Broader benefits and biosimilars will offset hefty fees and discounts while preserving R&D incentives.