
As the biopharma industry awaits FDA’s guidance on biosimilar naming, brand and generic manufacturers establish positions.

As the biopharma industry awaits FDA’s guidance on biosimilar naming, brand and generic manufacturers establish positions.

Physician confidence in biosimilars and extrapolation of indications are two of the most important factors governing the acceptance and sustainability of biosimilars, say industry panelists.

The agency publishes draft guidance answering industry questions about the Biologics Price Competition and Innovation Act.

The US Court of Appeals granted Amgen’s request to block Novartis’ Neupogen biosimilar, Zarxio, from the US market until the court resolves litigation between the two companies.

Eleven of the leading US biosimilar developers have collaborated to form the Biosimilars Forum, a nonprofit organization formed to expand patient access to biosimilars.

Intellectual property lawyers estimate biosimilar litigation will swell as early as 2018.

FDA releases long-awaited guidance documents regarding the assessment of biosimilarity.

Originator product manufacturers will have to update and improve their processing platforms to stay competitive with the biosimilars coming to market.

The company announced that a pharmacokinetic study comparing ONS-1045 to US- and EU-sourced Avastin will conclude shortly.

Biosimilar applicants will not be required to hand over their biosimilar applications and manufacturing dossiers to innovator companies, determines FDA.

As a result of the decision, Sandoz will be able to immediately launch Zarxio, the first biosimilar in the United States.

BIO and the Colorado BioScience Association urge Colorado Governor Hickenlooper to sign a bill that will help patients gain access to interchangeable biologic products following FDA approvals.

FDA approved Sandoz’s Zarxio (filgrastim-sndz) on March 6, 2015. The approval is a groundbreaking decision, as Sandoz is the first pharmaceutical company to have a biosimilar product approved in the United States. Known as Zarzio outside of the US, Sandoz says its biosimilar filgrastim is already available in more than 60 countries worldwide, has generated more than 7.5 million patient-days of exposure, and is "the most widely used filgrastim in Europe."

FDA announced that it would postpone a meeting that would be critical for the advancement of Celltrion’s Remicade biosimilar in the US.

Remsima will now be available for patients in 12 additional countries in the European Union.

The Patent and Trademark Office sends notice rejection of the company’s Remicade patent.

Hospira’s Inflectra (infliximab), a biosimilar for Remicade, is approved by the European Commission.

The National Institute for Health and Care Excellence released an updated version of its biosimilar approach guidance, including increased consideration for technology appraisal, references in documentation, and the production of “evidence summaries”.

Pfenex announced that it entered into an agreement to partner with Hospira on the development and commercialization of PF582, a biosimilar candidate to Lucentis.

On February 3, 2015, the FDA published a notice in the Federal Register that it is soliciting input on the collection of data to support interchangeability claims in biosimilar applications.

Amgen announced that it met primary and secondary endpoints in its biosimilar evaluation of adalimumab for the treatment of rheumatoid arthritis, when compared to Humira.

The deal may offset billions of dollars in waning sales from Pfizer drugs slated to lose patent protection and provides Pfizer with a whole portfolio of biosimilar products.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.

Market forces may limit the success of CMOs.
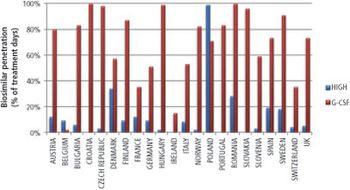
Investors are lining up for the biosimilars market as patents reach expiration and regulatory pathways are defined.