
The company announced plans to begin PIONEER, a Phase IIIa program comprising seven trials of approximately 8000 people with type 2 diabetes.

The company announced plans to begin PIONEER, a Phase IIIa program comprising seven trials of approximately 8000 people with type 2 diabetes.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

A vaccine patch may eliminate the need for traditional means of vaccine distribution, according to an article in NPR.

Ethylene vinyl acetate (EVA) drug-release technologies are explored.

Recently published research demonstrates how nanoparticles can be used to overcome hurdles in localized drug delivery.

A team from Northwestern University has demonstrated the feasibility of topical delivery of small interfering RNA (siRNA).

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

This article discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.
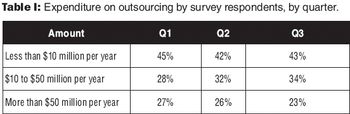
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.
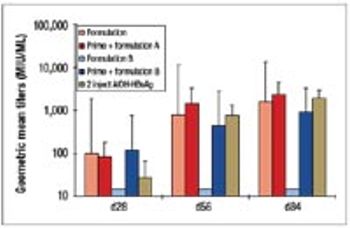
The disadvantages of the traditional vaccine regime (prime plus boost) have spurred the development of single-shot vaccines. This article describes the development and manufacture of a prototype single-shot vaccine that uses microspheres made from cross-linked modified dextran polymers for controlled release of the antigen.

The transdermal delivery of biologics-as well as of conventional drugs-is growing in popularity because the technique offers numerous advantages.