
Technology advances enable contract service providers to keep pace with the demands of existing and emerging biologic-based therapies.

Technology advances enable contract service providers to keep pace with the demands of existing and emerging biologic-based therapies.

Brammer Bio establishes late-phase development and commercial manufacturing facility for advanced cell and gene therapies in Lexington, MA.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

Use of a subspace model is a viable method to characterize process space variables and optimize process performance.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

Cytovance Biologics anticipates continued expansion plans following acquisition by Hepalink USA

CMC Biologics and River Vision Development announce manufacturing agreement for RV001, a monoclonal antibody to treat Grave’s orbitopathy.

Catalent’s licensing of Excelimmune’s antibody combination therapy platform can enable more consistent, cost effective production of antibody combinations.

Lonza’s planned facility will be used to develop and manufacture viral gene therapies and virally modified cell therapies

Rentschler Biotechnologie launches 2000-L single-use bioreactor and announces additional expansion.

CMOs may find opportunities in alternative expression services.

With numerous biologics set to come off patent soon and the percentage of new therapeutics based on biomolecules growing, demand for contract manufacturing in the biopharma space is heating up.

Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Growth is seen in outsourcing of insect- and plant-cell-based bioproduction expression systems.

Formulators and developers are at the heart of the industry's basic premise-they are saving lives.
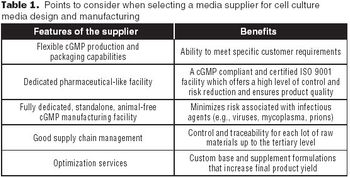
To select the right partner for media design and optimization services, several key factors must be considered.
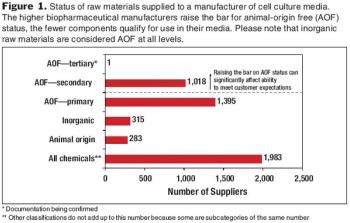
How to successfully balance patient safety with supply-chain management
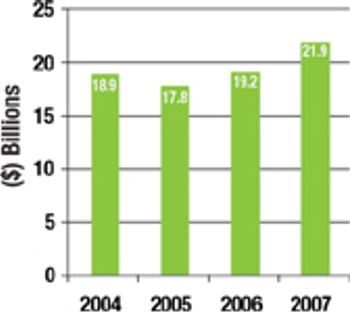
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.
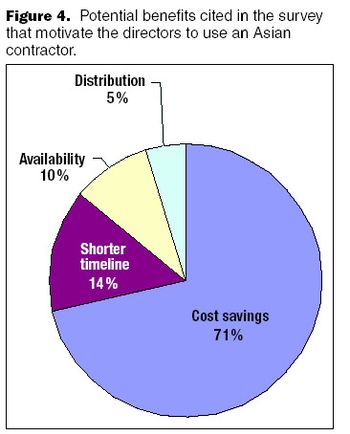
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

Cobra Biomanufacturing is an international full-service manufacturer of biopharmaceuticals, dedicated to designing robust processes that deliver biopharmaceutical products to its life sciences customers for preclinical through Phase 3 studies.

There wasn't much of a contract services industry when BioPharm International began publishing 20 years ago. Today's big names in biomanufacturing, including Lonza, Boehringer-Ingelheim, and Avecia, had not yet entered the business.
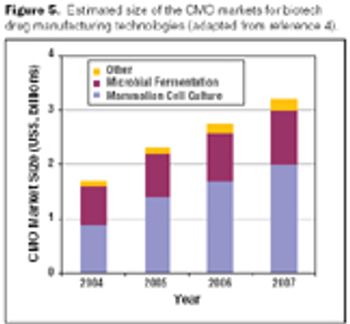
Biologics manufacturing is a technologically complex, highly regulated process. In comparison to small-molecule manufacturing, biologics manufacturing requires far more planning, investment, and skilled personnel and, therefore, can be much riskier. For biotech companies requiring such manufacturing capabilities and experience, partnering with a biologics-focused contract manufacturing organization (CMO) can be a good solution.

The three largest players have accumulated, or are in the process of accumulating, nearly a million liters of capacity between them.