
The authors describe the qualification of an assay with applications for investigating functional comparability of an originator and biosimilar drug.

The authors describe the qualification of an assay with applications for investigating functional comparability of an originator and biosimilar drug.

EAG Laboratories announces new company identity and intent to expand testing, analysis, and characterization capabilities across multiple markets.

Technology advances enable contract service providers to keep pace with the demands of existing and emerging biologic-based therapies.

Contract service providers debate the pros and cons of consolidation, accelerated drug approvals, regulatory issues, and the impact of new therapies.

Investment at SGS’s Mississauga, Canada facility provides for analysis of molecular interactions in real time.

The authors investigate the sufficiency of high-temperature short-time treatment in inactivate mouse minute virus contamination.

AMRI adds analytical capabilities to its outsourcing services offerings with the acquisition of Whitehouse Labs.

The testing laboratory will add capacity to strengthen its early-stage drug research capabilities for its small and large biotech clients.
Focusing on niche and specialty service offerings gives contract biomanufacturing organizations an opportunity to differentiate in a crowded market.

WuXi's Laboratory Testing Division will be the exclusive supplier of laboratory testing services for Hong Kong-based Lee's Pharm.

Malvern Instruments' biophysical characterization equipment will be installed in a commercial applications laboratory in San Diego, California.

Evans Analytical Group expands into the pharmaceutical/biopharmaceutical industry with acquisition of ABC Laboratories.

A new SGS Life Science Services laboratory outside Paris is designed for bio/pharmaceutical quality control testing.

Contract service providers share insights on biopharma market developments and the implications of biosimilar drug approvals.

The rapid testing of biologic raw materials can lead to greater efficiency.

The agency outlines recommendations for the development and submission of near infrared analytical procedures.
It is important to understand degradation and processing to maintain product stability in biologics.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.
Ligand-binding assays are fundamental to characterizing biosimilars.

A Q&A on bioanalytical method integrity with Roger Hayes of MPI Research.

Through its educational and networking opportunities, the American Association of Pharmaceutical Scientists plays an important role in partnering throughout the drug- development and commercialization process.

By following key strategies, companies can reduce the risk and increase the benefits of outsourcing analytical development and testing

It is essential to build and maintain a good working relationship between the client and contract research organization.
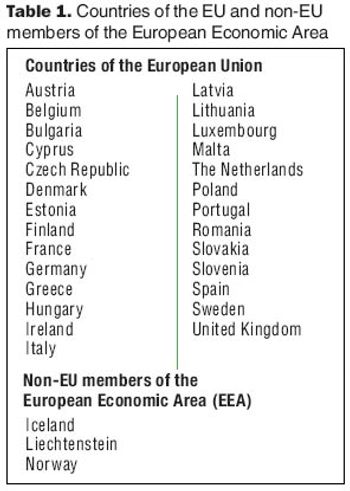
A good understanding of European regulations governing batch release testing will facilitate your collaboration with a contract laboratory.

Communication between contract analytical laboratory and client is extremley important when out-of-specification (OOS) results arise.