
Merck relaunches brand identity to reflect transformation into a science and technology company.

Merck relaunches brand identity to reflect transformation into a science and technology company.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

Avantor released a packaging platform that allows for the direct dispensing of chemicals-such as salts, buffers, and cell culture ingredients-into processing equipment.

The process provides an alternative to the sterilization of cartridges and vials with ethylene oxide (EtO).

Hiroaki Suzuki will lead Vetter’s business activities in the new Tokyo-based office.

Althea is expanding its existing biological drug product manufacturing operations to include highly active materials, such as antibody-drug conjugates, in a new facility near San Diego, CA.

The company presented a portfolio of new products during the meeting in Madrid.

The new sugars are touted to increase the efficacy of active pharmaceutical ingredients and improve cell culture yield.

The Bothell, WA location will help the company reach its goal of expanding its global capacity by more than 30,000 liters.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

Merck Millipore’s collaboration with celares GmbH launches pegylation services for protein-based therapeutics.

Vetter plans to invest approximately 300 million Euros during the course of five years to expand drug product manufacturing and logistic services in Germany; upgrades will include an improved RABS system for aseptic processing.

Pharma development and manufacturing firm Patheon announces new tagline and logo.

Novo Nordisk will build a local manufacturing plant for FlexPen prefilled insulin delivery devices in Iran.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

Wilden’s Saniflo Hygienic Series pumps use a new, energy-efficient air distribution technology.
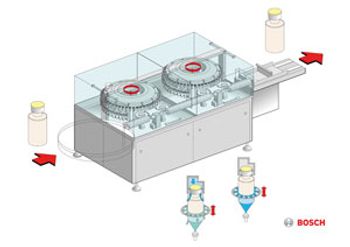
The RAN 3080 exterior washing machine from Bosch cleans filled and closed vials, ampuls, and cartridges using a high-pressure process and special transportation system.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

The platform, developed by Vectron Biosolutions, will be used for BI’s in-house pipeline and for its outsourcing clients.

The collaborative effort will be focused on fully humanized antibodies.

Under the terms of the agreement, Sartorius Stedim Biotech will manufacture membrane adsorber technologies for GE, which will be marketed as part of GE’s ReadyToProcess product offerings.

WuXi's Laboratory Testing Division will be the exclusive supplier of laboratory testing services for Hong Kong-based Lee's Pharm.

Cytovance Biologics anticipates continued expansion plans following acquisition by Hepalink USA

The acquisition will give IDT Biologika access to the veterinary vaccines market.

The partnership will help divert 840 tons of waste related to single-use products from landfills or incineration during the next year alone.

CMC Biologics will manufacture monoclonal antibodies (mAbs) and provide process development services for the PATH Malaria Vaccine Initiative.

The platform detects the virus through silver nanoparticles attached to antibodies.

Following a divestiture of certain assets in Europe, the acquisition of Sigma-Aldrich by Merck KGaA will be complete.

This new range includes antibodies conjugated to various enzymes, fluorescent dyes, and colloidal gold, for improved flexibility in experimental design.

Flexion will have a dedicated manufacturing suite at Patheon's Swindon, England facility for Flexion's osteoarthritis injectable steroid drug product.