
The company will be presenting at CPhI Worldwide on Oct. 11, 2018.
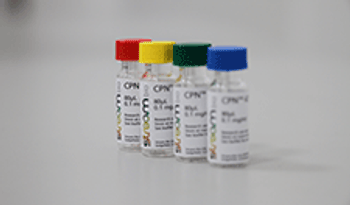
The companies have partnered to launch Conjugated Polymer Nanoparticle (CPN) products for use in molecular imaging and R&D applications.

The drug particle engineering and nanotechnology company offers a nanotechnology platform that can revive failed drugs in the pharma pipeline.

PCI Pharma Services and CSP Technologies will partner on protective packaging solutions for clinical trials and stability testing.

The provider of plant-based ingredients will present recently launched multi-compendial materials for upstream and downstream biopharmaceutical applications.

Avara Pharmaceutical Services acquired Sandoz’s sterile manufacturing facility for injectable medicines in Boucherville, Quebec, Canada.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

The collaboration will focus on developing manufacturing solutions for biosimilars.

Gore’s new flexible freeze containers are designed to protect high-value drug substances from container breakage or leakage.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

ICS distribution center will serve manufacturers of specialty medications, biosimilars, and cell and gene therapies.

Becton Dickinson’s (BD) Advanced Bioprocessing business will be integrated into Thermo Fisher's Life Sciences Solutions segment.

Lonza’s new PyroTec Pro Robotic Solution provides a fully automated workflow for endotoxin detection.

The pharmaceutical, clinical, and bioanalytical contract solutions provider has implemented advanced techniques for the collection and use of peripheral blood mononuclear cells (PBMCs) for early-phase clinical trials at its Clinical Pharmacology Unit in Antwerp, Belgium.

The company is increasing manufacturing capacity at its Copenhagen, Denmark facility with the addition of six new bioreactors.

A collaboration integrates Sartorius Stedim Biotech’s BIOSTAT STR bioreactors and Repligen’s XCell ATF cell-retention control technology to create simplified, scalable equipment for intensified cell culture.

Alcami and the University of North Carolina Wilmington received a grant from the National Institute for Innovation in Manufacturing Biopharmaceuticals to prepare students for careers in pharmaceutical sciences.

Flush solutions for liquid chromatography mass spectrometry (LC-MS) systems and ultrapure solvents for ultra-high-performance LC-MS systems from Thermo Fisher Scientific can minimize interference and maximize lab instrument uptime.

Protagen Protein Services, a CRO, now offers quicker and more accurate characterization of biomolecular stability using differential scanning calorimetry (DSC).

Shimadzu’s Imagereveal MS mass spectrometry imaging data analysis software can analyze large sets of data or simultaneously analyze multiple sets of data.

The acquisition strengthens Nelson Labs’ outsourced testing capabilities for the pharmaceutical and medical device industries.

The TSQ Fortis Triple Quadrupole Mass Spectrometer from Thermo Fisher Scientific offers fast, robust liquid chromatography-mass spectrometry (LC-MS/MS) analysis for clinical research laboratories.

The Lumis electron backscatter diffraction (EBSD) detector incorporates a large-format complementary metal oxide semiconductor (CMOS) sensor to enable higher sample throughput, higher resolution measurements, and precise phase identification.

The company is certified as a manufacturer of pressure vessels and components for use in China.

An expansion of laboratory facilities in Geneva, Switzerland expands SGS services for high-order structure analysis.

The new Testa Center in Uppsala, Sweden is a collaborative test bed offering biotechnology equipment from GE Healthcare for process development.

The acquisition is expected to support Emergent BioSolutions’ focus on public health threats and emerging infectious disease.

Pharmaceutical scientist association announces upcoming term’s board of directors.

Drug product approval from FDA follows previous approvals from European and Japanese authorities.