
Alternative materials, as well as supply-chain planning, are essential to ensure a reliable supply for parenteral drug packaging.

Alternative materials, as well as supply-chain planning, are essential to ensure a reliable supply for parenteral drug packaging.
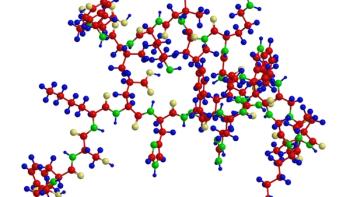
Establishing analytical workflows for complex biopharmaceutical molecules can help predict their risk of degradation.

Advancements in bioprocessing technologies test microbial fermentation adaptability.

Process chromatography in downstream processing has seen steady improvements over the years, but there is still room for improvement.

The industry considers applying automation and digitalization lessons learned during the COVID-19 pandemic to enhance workflows.

Mechanistic models provide process understanding for developing robust manufacturing processes and for scale up and tech transfer.

Models of biopharmaceutical processes can be used for speeding development and improving process control.

As interest in cell and gene therapies continues to grow, the need for safe and consistent reagents to support research, development, and manufacture efforts also increases.

BioPharm International asked Vincent Colicchio, vice president, supply chain and external manufacturing at Dr. Reddy’s Laboratories, about how a lack of proper supplier oversight can impact the pharmaceutical industry and the drug supply chain.
Scientific advances open the way to alternatives to animal testing and in-vivo assays.

Almost half of pharmaceutical industry profits continue to come from biopharmaceuticals.

Will FDA’s approval of Semglee create a surge in the development of interchangeable biosimilars?

Siegfried Schmitt, vice president, Technical at Parexel, provides best practices for switching from paper-based to automated processes.

The mission continues. Let science be the guide.