
Implementing Toyota’s LEAN Systems in Bioprocessing

Implementing Toyota’s LEAN Systems in Bioprocessing
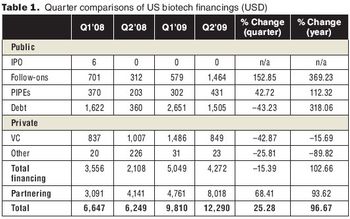
Biotech impressed investors with positive drug data, strong drug sales and earnings, and partnering deals.

Ten biopharmaceuticals gained marketing authorization in the US or EU in 2008, although only four were new approvals.
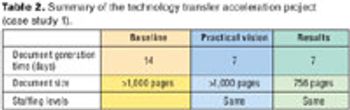
A step-by-step approach is essential for successful implementation.
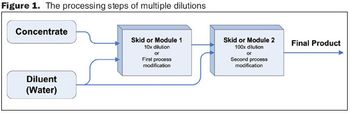
Automated in-line dilution can help solve capacity, financial, and quality concerns that biopharmaceutical manufacturing plants may be facing.

It seems clear that insuring the roughly 46 million Americans who are now uninsured will increase drug sales.

Small changes can have a big effect further downstream in your manufacturing processes.

Lonza's bid for Patheon makes the contract manufacturing industry re-examine the one-stop manufacturer model.

Stiffer enforcement of quality standards aims to restore public confidence in agency actions.