
Michiel E. Ultee of Laureate Biopharmaceutical Services gives an update on 1988 article regarding virus testing and how the advance of monoclonal antibodies has changed processes.

Michiel E. Ultee of Laureate Biopharmaceutical Services gives an update on 1988 article regarding virus testing and how the advance of monoclonal antibodies has changed processes.

Ties between the biotechnology industry and university research are crucial.

Using a competency-based approach to effectively train biopharmaceutical industry staff.
![JimMiller-D-[114739850]-787740-1408598506821.jpg](https://cdn.sanity.io/images/0vv8moc6/biopharn/5ec65520a609e4c058df4707c47d1c52ce19a4d1-123x150.jpg?w=350&fit=crop&auto=format)
The weak global economy adds to the challenges of bio/pharma companies and their suppliers.
The authors describe a validation master plan for closed-vial filling technology.

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.
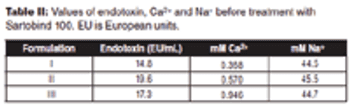
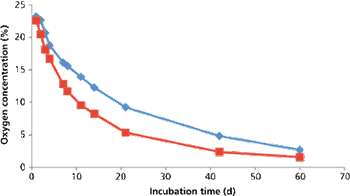
The authors describe a simple method to remove endotoxins from highly viscous formulations.

A perspective on why platform processes are not and should not define the future of bioprocess development efforts. This article is part of a special section on biopharmaceutical trends.

NIH has awarded ten laboratories two-year grants to develop tissue chip technology, with part of the funding coming from the recently established National Center for Advancing Translational Science.

PDA's strategic plan calls for maintaining valuable relationships with global regulators.

NIBRT's Ian Nelligan on what to expect when starting an upstream process, including the choice between single-use and stainless-steel bioreactors.

Brazil's regulatory health authority, Anvisa, plans to establish quality requirements for locally produced pharmaceutical excipients, Anvisa told BioPharm International.

Howard Levine of BioProcess Technology Consultants talks about what industry needs to know to enter the biosimilars game in the US.

Manufacturers and regulators struggle to control phony versions of crucial medicines.