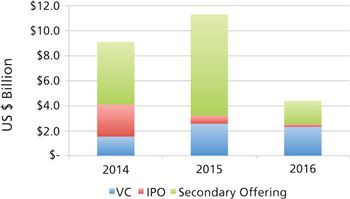
CDMOs need to be aware that unfavorable public markets put emerging bio/pharma R&D spending at risk in 2017.

CDMOs need to be aware that unfavorable public markets put emerging bio/pharma R&D spending at risk in 2017.

Revised versions of ISO 14644 Parts 1 and 2 introduce changes to sampling procedures and monitoring plans for cleanrooms and clean zones.

The authors present an overview of the types of RNA-based therapeutics in existence and their optimal methods of manufacture, purification, formulation, and delivery.

Recent trends in raw materials packaging may impact manufacturing, quality, and cost of biopharmaceuticals.

The author provides a review of the concepts of design and qualification that apply to single-use systems.

Capacity for complex therapeutics is become increasingly difficult to predict.

Pressures to accelerate current and next-gen therapies are challenging traditional microbiological testing methods.

CPhI Pharma Awards seek nominations for excellence in biopharma development and manufacturing.

The ambr 250 mini benchtop bioreactor system from Sartorius Stedim Biotech can be used for parallel fermentation and cell culture.

Agency guidance and industry standards aim to reduce lapses and improve quality operations.
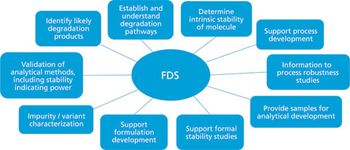
The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.

Click the title above to open the BioPharm International July 2016 issue in an interactive PDF format.