This article reviews the definition of HCPs, risks posed by HCPs, regulatory concerns, commonly accepted ELISA methods for HCP measurement and their limitations, and orthogonal methods available for HCP characterization.

This article reviews the definition of HCPs, risks posed by HCPs, regulatory concerns, commonly accepted ELISA methods for HCP measurement and their limitations, and orthogonal methods available for HCP characterization.
Single-use and modular technologies plus continuous manufacturing are increasingly important to biopharma scale-up and tech transfer.

Will biosimilars share a compendial identity like generic drugs do?

Removal of microorganisms is crucial when working with biologics. Sterile filtration offers a reliable, safe, and effective way to ensure product integrity.

Regulators and industry seek to streamline and harmonize oversight of postapproval changes.
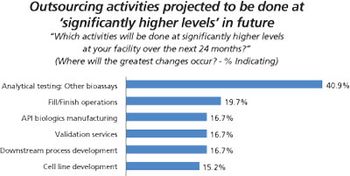
Biopharma companies on both sides of the Atlantic ship more of their assay testing to outside service providers.

Meritech’s CleanTech 2000SCA Automated Handwashing System delivers a 12-second wash and rinse cycle that removes 99.98% of dangerous pathogens from bare skin and gloves.

Hamilton’s EasyFerm Bio biocompatible pH sensor is designed for CIP, sterilization up to 140 °C, autoclavable temperature, and a pressure range up to 6bar.

Objective, peer-reviewed papers and technical articles can help advance biopharmaceutical development.

Click the title above to open the BioPharm International June 2015 issue in an interactive PDF format.

The Australian pharmaceutical market offers opportunities for manufacturers despite challenges.
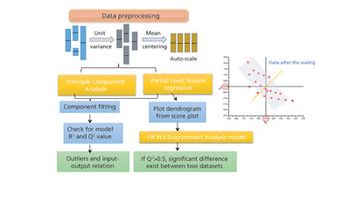
The authors review major developments in use of MVDA in bioprocessing applications.