
Jessica Rousset, chief operating officer, CURE Pharmaceutical, highlights some key regulatory considerations developers should keep in mind when approaching alternative dosage forms.

Jessica Rousset, chief operating officer, CURE Pharmaceutical, highlights some key regulatory considerations developers should keep in mind when approaching alternative dosage forms.

In light of recent FDA guidance on data integrity, the challenges and benefits of using the public cloud to deploy and use data management software are discussed.

Technology vendors are strengthening their positions in China as the nation emphasizes self-sufficiency in research, development, and manufacturing.
Single-use systems can be a cost savings for CMOs, and these savings can be passed on to clients and, ultimately, to patients.

FDA expects more than 200 investigational new drug applications for cell and gene therapies by 2020, causing the agency to strengthen its regulatory program.

Optimizing the patient experience and technological advances can positively impact adherence.
Both empty and filled syringes must pass a range of tests to meet quality standards for biopharmaceutical drugs.

Simplification is crucial to maintain data integrity, according to Siegfried Schmitt, PhD, vice-president, technical at PAREXEL Consulting.

Do patients get what they pay for when they demand cheaper drugs?

A properly designed validation program will detect variation and ensure control based on process risk.

A major advantage of SPR-based analysis is its ability to estimate the association and dissociation rate constants, an advancement over the traditional steady-state analysis of biomolecules.
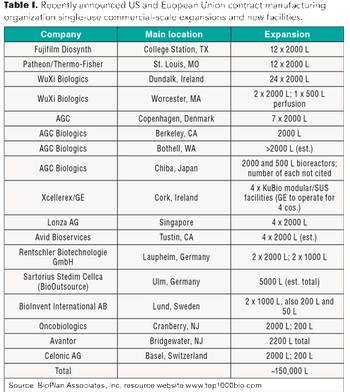
A look at recent investments by contract manufacturers to increase single-use bioreactor capacity.

Downstream process equipment for mAbs manufacturing must be designed to fit technology developments in upstream processes.

As automation in biomanufacturing becomes more important, so does the need to integrate process data.

Click the title above to open the BioPharm International March 2019 issue in an interactive PDF format.