
The companies aim to assess automated CAR-T cell therapy manufacturing at the point-of-care and develop technologies to facilitate patient access to immunotherapies.

The companies aim to assess automated CAR-T cell therapy manufacturing at the point-of-care and develop technologies to facilitate patient access to immunotherapies.

The companies announced a collaboration to jointly discover and develop treatments to address unmet medical needs in a specific neurological disease.

The new facility, located at the company’s headquarters in Pittsburgh, PA, is expected to meet all clinical and commercial development needs of the company’s lead gene therapy program.

The partners will collaborate on the development and commercialization of a B-cell maturation antigen-targeting immunotherapeutic for treating multiple myeloma.

The companies will jointly develop and commercialize an investigational bifunctional fusion protein immunotherapy currently in clinical development for cancer treatment.

Horizon and Rutgers University will partner to develop and commercialize new gene editing technology for therapeutic and research applications.

The agency revised draft guidance to enable more efficient and successful drug development programs for the treatment or prevention of rare diseases.

Researchers at the University of Delaware have made a step forward in gene therapy by engineering microparticles that deliver gene-regulating material to hematopoietic stem and progenitor cells, which live deep in bone marrow and direct the formation of blood cells.

A new gene therapy can turn certain brain glial cells into functioning neurons, which could help repair the brain after a stroke or during neurological disorders like Alzheimer’s or Parkinson’s diseases, according to researchers at Penn State.

AbbVie and immunotherapy company Tizona Therapeutics will join forces to develop and commercialize CD39-targeted therapeutics to treat cancer.

Agilent and other partners are funding development of Tapestri, a single-cell sequencing platform designed to help predict cancer relapse in individual patients and show the efficacy of gene-editing experiments.

The companies aim to advance research into inflammatory bowel disease.

FDA has approved Truxima (rituximab-abbs), a biosimilar to Roche’s anti-cancer biologic, Rituxan (rituximab).
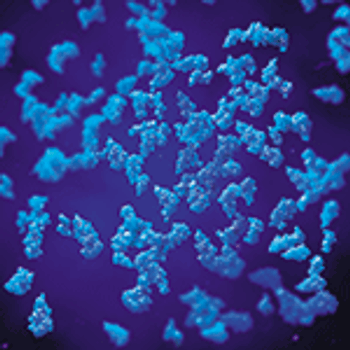
This research proposes a method for separating and purifying tissue-type plasminogen activator from a fungal cell source.

The acquisition will give Genentech full rights to Jecure’s preclinical portfolio of NLRP3 inhibitors.

The companies will develop AMG 714, a novel anti-IL-15 monoclonal antibody, for the treatment of gluten-free diet non-responsive celiac disease in a collaboration worth $170 million.

More-and earlier-interaction between R&D and payers will be essential if innovative therapies are to become more accessible for patients, and more profitable for manufacturers, said panelists at the 2018 Galien Foundation Forum.

AbbVie will assume full development and commercial responsibility for its collaboration with Galapagos to discover and develop new therapies to treat cystic fibrosis (CF).

The companies will work together to advance a number of Morphic's oral integrin therapeutics for fibrosis-related indications in a deal with $100 million.

The companies will work together to develop Ionis-FB-LRx for the treatment of complement-mediated diseases, including Geographic Atrophy (GA), the advanced stage of dry age-related macular degeneration (AMD), in a deal worth $760 million.

Novartis plans to acquire Endocyte, a biopharmaceutical company, to accelerate the development of innovative radioligand technology for treating cancer.

Roche has acquired Tusk Therapeutics in a deal worth up to EUR 659 million (US$762 million).

The companies will work together to discover, develop, and commercialize immunotherapies for patients with solid-tumor cancers in a collaboration worth $695 million per program.

The National Institute for Health and Care Excellence recently rejected National Health Service’s funding of Kymriah for diffuse large B-cell lymphoma (DLBCL) despite recognizing that the drug has significant clinical benefits.

The European Medicines Agency has recommended Luxturna (voretigene neparvovec) as the first treatment option for hereditary retinal dystrophy with mutations of the RPE65 gene.