
Smaller biopharma companies must do due diligence with their CMC strategy for BLA filings.

Smaller biopharma companies must do due diligence with their CMC strategy for BLA filings.

The evolution of regulatory guidelines for biosimilars alongside improvements in knowledge and understanding provide a platform for growth in the European market.

Enzyvant’s Rethymic is the first FDA approved treatment for congenital athymia.

Roche has submitted a marketing authorization application to EMA for Ronapreve (casirivimab/imdevimab) as a treatment for COVID-19.

EMA has halted the rolling review of CureVac's COVID-19 candidate, CVnCoV, due to the company withdrawing from the process.

MHRA has awarded an ‘Innovation Passport’ to CellResearch for its umbilical cord lining stem cell therapy, CorLiCyte.

The approval of ChemoCentryx’s Tavenos (avacopan) provides a new treatment for active anti-neutrophil cytoplasmic autoantibody-associated vasculitis.

AstraZeneca’s tezelpelumab was granted orphan drug designation by FDA for the treatment of eosinophilic esophagitis.

FDA’s emergency use authorization will double rapid at-home COVID-19 testing capacity.

The agency received a marketing authorization application a monoclonal antibody treatment for COVID-19 patients.

The agency has concluded that an extra dose of the COVID-19 vaccines may be given to those individuals with severely weakened immune systems.
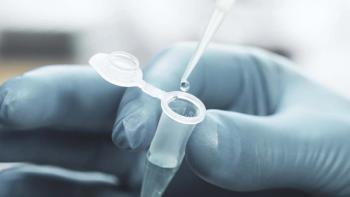
Establishing bioassay studies for biosimilar development is important for supporting regulatory filings.

A new program will test the safety and suitability of new inactive ingredients to encourage the accelerated adoption of FDA-accepted excipients in drug development.

FDA granted orphan drug designation to Catalyst Biosciences’ MarzAA for the treatment of Factor VII Deficiency.

FDA approved AbbVie’s Qulipta (atogepant) as a preventative migraine treatment.

GeneTx Biotherapeutics and Ultragenyx Pharmaceutical announced that FDA has removed the clinical hold on GTX-102, an investigational treatment for Angelman syndrome.

Biosimilar approvals and the advance of biosimilar testing and production may lead to greater access to alternative therapies.

The agency approved Byooviz (ranibizumab-nuna), a biosimilar to Lucentis (ranibizumab injection), for the treatment of several eye diseases.

Pfizer-BioNTech announced plans to seek FDA approval this month of a smaller dose of its Comirnaty vaccine for children ages 5–11.

FDA advisors agreed unanimously on a more limited booster plan after rejecting Pfizer’s original request to authorize its third shot for everyone over age 16.

Health Canada approved the new drug submission for Spikevax—more commonly known as the Moderna COVID-19 vaccine—for active immunization to prevent COVID-19 in individuals 12 years of age and older.

FDA revised the emergency use authorization for bamlanivimab and etesevimab (administered together) to include emergency use as post-exposure prophylaxis (prevention) for COVID-19.

Takeda’s Exkivity (mobocertinib) was approved by FDA for the treatment of patients with lung cancer.

FDA and EMA will provide scientific advice to applicants concerning complex generic and hybrid products.

HUTCHMED’s amdizalisib (HMPL-689) received breakthrough therapy designation for the treatment of a subtype of non-Hodgkin’s lymphoma.