
New formulations and expanded vaccine production are encouraged.

New formulations and expanded vaccine production are encouraged.

FDA revises interpretation of the five-year NCE exclusivity provisions for certain fixed-combination drug products.

Genzyme plans to appeal FDA?s decision that the multiple-sclerosis treatment is not ready for approval.

The JOBS Act and FDASIA show early signs of accelerating drug development.

Zhoydro ER is the first drug to have updated labeling now required for all ER/LA opioid analgesics.

FDA funds research to further development of innovative generics, while working to address review and approval issues.

FDA has released Guidance for Industry: Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination.
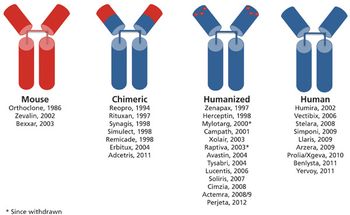
Innovative products and a range of indications drive the therapeutic antibody market.

Additional approvals in December have helped to outpace a recent high set in 2011.

Therapeutics targeting epigenetic mechanisms of disease will change the pharmaceutical marketplace.

Will the next US President support the backbone of our industry?

Howard Levine of BioProcess Technology Consultants talks about what industry needs to know to enter the biosimilars game in the US.

Companies often wait for a critical mass before adopting new technologies. But if no one takes the risk, critical mass will never be reached.

The FDA is launching a pilot program to allow manufacturers to electronically file drug establishment registration and drug listing information, such as ingredients, labeling, and manufacturing information.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.

More informed submissions may lead to regulatory flexibility for postapproval changes.

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) issued a revised draft guidance on July 20 to help ensure that the safety, purity, and potency of biologics products is not compromised as a result of innovative, flexible manufacturing arrangements.

Utility patents are granted to anyone who invents any new and useful process, machine, article of manufacture, composition of matter, or any new improvement thereof.

Human infections with avian flu strain H5N1 are occurring in a number of southeast Asian countries that have experienced large outbreaks of avian influenza. How great a risk to the human population is posed by this virus, and what steps can be taken to minimize its impact? Preventive vaccines have great potential to avert the spread of avian flu and other infectious diseases. What are the factors affecting the creation of new vaccines, and how can they be optimized to promote public health?