
The European Medicines Agency has begun a rolling review based on laboratory and clinical studies of the Sputnik V (Gam-COVID-Vac).

The European Medicines Agency has begun a rolling review based on laboratory and clinical studies of the Sputnik V (Gam-COVID-Vac).

The guidance document provides recommendations for reporting and implementing changes to container closure system components consisting of glass vials and stoppers for sterile drug products.

The need for real-time monitoring and control has spurred the development of new analytical tools.

Accelerated drug development timelines must accommodate all crucial elements of nonclinical safety studies.

Reviews of FDA initiatives and activities recapitulate efforts to assess and approve new drugs, generic drugs, and biologics and strategies for advancing new initiatives.

Auditing distribution suppliers provides understanding and documentation of the services performed.

To prevent future production delays of critical products, the Biden administration is examining supply chain vulnerabilities for pharmaceutical ingredients as part of a longer-range consideration of products important to public health.

The agency issued guidance for developers of vaccines, diagnostics, and therapeutics to address variants of the COVID-19 virus.

FDA issued a public statement and press release, along with a sharp warning letter rebuking AcelRx Pharmaceuticals for its messaging on painkiller Dsuvia.

FDA published recommendations on the use of COVID-19 convalescent plasma or investigational convalescent plasma during the pandemic.

The EC has granted marketing authorization to Celltrion Healthcare for its adalimumab biosimilar, Yuflyma (CT-P17).

Scientists and policy makers are looking more sharply at the lag in identifying and producing medicines to moderate early infections and to treat seriously ill patients.

The EC has granted Vico Therapeutics with orphan drug designation for its investigational antisense oligonucleotide therapy for the treatment of SCA.

EMA has clarified its position on the European approval process of the Sputnik V vaccine.

The agency is developing guidance to help manufacturers make changes to existing COVID-19 vaccines to treat new variants in the coronavirus.

The selection of a new FDA commissioner has become a contentious issue in Washington, as the White House delays a decision and interest groups line up behind competing candidates.

Pharma companies find ways to maintain quality standards, despite pandemic-related restrictions.

FDA considers regulatory flexibility to allow the agency and sponsors to adapt and pivot to changes in coronavirus.

The company recently installed a fully validated visual inspection system that uses artificial intelligence in an automated inspection machine.

PathoQuest will apply its experience in viral safety testing and quality control of biologics for mitigating the risk of adventitious agent contamination in biopharmaceutical manufacturing.

A task force details its findings of the risks associated with using convalescent plasma using failure mode and effects analysis
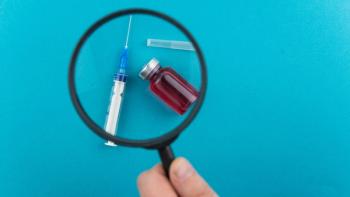
Amid the rush for a SARS-CoV-2 vaccine to deal with the COVID-19 pandemic, a robust risk assessment must be conducted, and mitigation strategies applied.

With appropriate planning and the proper use of technology, remote auditing can be as effective and informative as in-person auditing.

FDA puts applications on hold as the agency limits alternative oversight methods.

With Democrats controlling Congress and the White House, expectations are high that policy makers will revise certain coverage and payment policies.