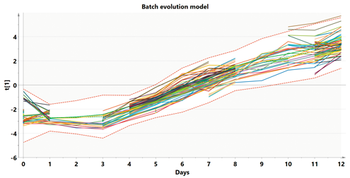
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

The study suggests that circumventing evolution in cell factories can enable the commercialization of new biobased chemicals to large-scale.

A collaboration between The Centre for Process Innovation and The Roslin Institute aims to develop commercially viable and scalable methods of producing biologics using transgenic animals.

The funding from the Walloon Region will enable Univercells to start developing a manufacturing platform aimed at driving down costs of biosimilars manufacturing.

The company has completed development of a first-generation production process for its chimeric antigen receptor regulatory T cell product portfolio and is selecting a CMO for clinical supply.

Miniature bioreactors add value by reducing validation efforts.

Use of a subspace model is a viable method to characterize process space variables and optimize process performance.

As Europe strives to firmly incorporate quality-by-design principles, there are several key issues that still need to be addressed.

EU authorities are stepping up their efforts to incorporate QbD principles.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.

A report commissioned by FDA evaluates the QbD paradigm.
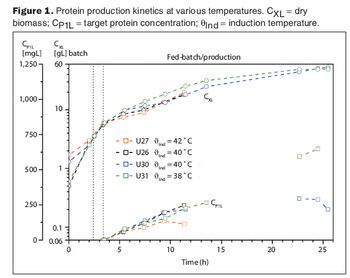
Two case studies show how advanced information technologies make process development more efficient.

Computational fluid dynamics can resolve performance problems.
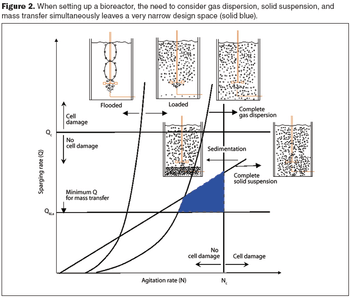
Computational fluid dynamics is a powerful tool to optimize processes.

One might look at QbD's plodding growth and conclude that it is never going to make it to graduation.

How will implementing Quality by Design strategies affect your compliance status?

The nimbleness of biotechs makes them well suited to implementing QbD. Here's how to get started.

Regulatory flexibility can make continuous improvement possible.
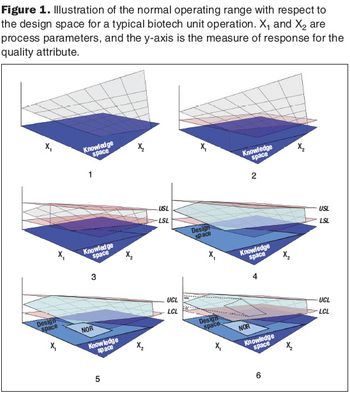
Second in a three-part series that discusses the complexities of QbD implementation in biotech development.

The focus on the design space will lead to a new workspace, and will affect staff in the development, manufacturing, and quality functions.
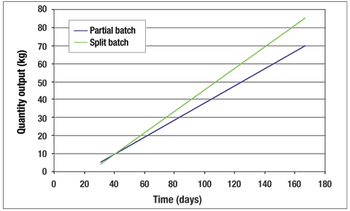
A case study in capturing indirect costs and benefits.

Without a rigorous discussion of the pros and cons of QbD, its tremendous benefits will be lost.

QbD for QA? Try a two-step approach

Understanding the relationship between the process and CQAs.