
This article reviews some of the commonly used approaches for process monitoring as well as the evolution of process monitoring in the Quality by Design (QbD) paradigm.

This article reviews some of the commonly used approaches for process monitoring as well as the evolution of process monitoring in the Quality by Design (QbD) paradigm.

Understanding the relationship between the process and CQAs.

No time for QbD? How to convince management to make it a priority.

The FDA's revised process validation guidance manages to explain the underlying concepts of Quality by Design without every using the phrase.
Using multivariate experiments to define acceptable ranges.
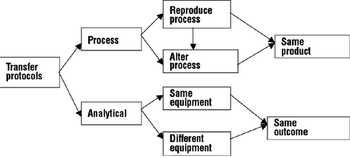
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.
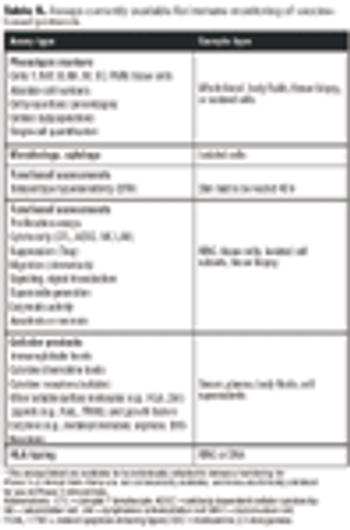
Emerging therapies pose challenges for standardizing QC.
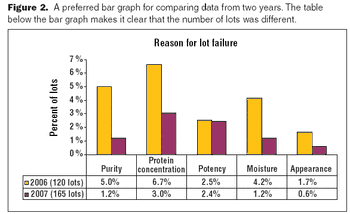
The key to a good graphical presentation is to select the method that best fits the data.

Multivariate data analysis can help biotech manufacturers deepen their process understanding.

Process development and manufacturing for biopharmaceuticals are often disjointed activities. Disconnects among groups are aggravated by a lack of common terminology and poor data management practices. A UK biotech consortium has initiated a collaborative development effort to address data management issues. The proposed outcome is a data model, based on the ISA-88 Standard for Batch Control, to capture process and facility data throughout the product lifecycle. A data framework that follows the ISA-88 model can simplify process scale up and enable early views of project costs and facility fit.

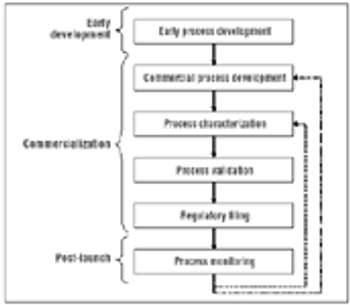
Process monitoring ensures that the process performs within the defined acceptable variability that served as the basis for the filed design space.
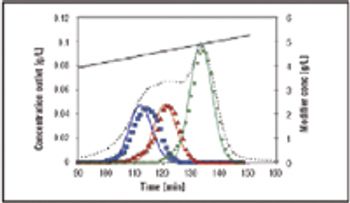
This article presents the multicolumn countercurrent solvent gradient purification (MCSGP) process, which uses three chromatographic columns, and incorporates the principle of countercurrent operation and the possibility of using solvent gradients. A MCSGP prototype has been built using commercial chromatographic equipment. The application of this prototype for purifying a MAb from a clarified cell culture supernatant using only a commercial, preparative cation exchange resin shows that the MCSGP process can result in purities and yields comparable to those of purification using Protein A.
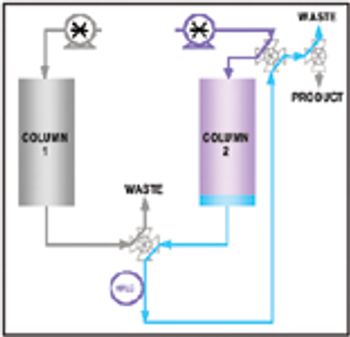
This article discusses how on-line high-performance liquid chromatography (HPLC) can measure product purity in the column eluent stream in near–real time. These data can then enable the automation and control of a purification column operation, thus reducing product variability, shortening process cycle time, and increasing yield. An example application demonstrates how on-line HPLC is used as a process analytical technology to ensure the process can accommodate variability in the separation while ensuring the product meets its critical quality attributes.

Invitrogen (San Diego, CA, www.invitrogen.com) believes that scaledown technologies can significantly improve clone selection and cell culture media development.

Increased resin stability can extend the number of cleaning cycles that can be performed in situ.

The challenge is to determine the optimal frequency for preventive maintenance and the optimal frequency and tolerances for calibration readings.

Increasingly, pharmaceutical companies have recognized that software development is not their core competency.
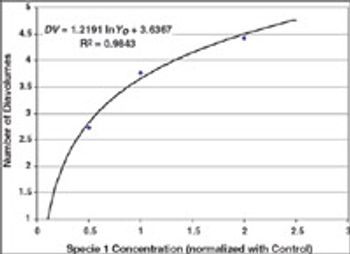
Case studies were run to test Process Analytical Technology applications for protein refolding, diafiltration, and cation exchange chromatography. It is shown that it is feasible to design control schemes that rely on measurement of product quality attributes and thereby enable real-time decisions.
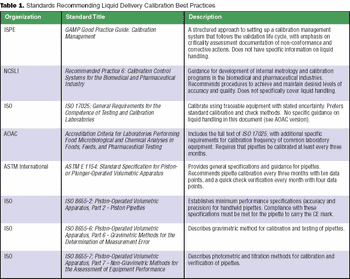
Federal regulations are broad and open to interpretation. Most have not caught up with advancements in technology.

For decades now, it has been said that "the process is the product" for biologics. Great care and consistency must be applied in their upstream manufacture-during fermentation, harvest, and early purification-to preserve their complex structure, which confers their activity and specificity. As the product moves to late-stage purification, however, the relative concentration of impurities and altered product forms is diminished. Also, the final dosage form of most large molecule biopharmaceuticals is the relatively simple liquid formulation of parenteral dosage form. In contrast, manufacturing the solid dosage forms common for small-molecule drugs involves more complex processes, such as mixing dry powders, granulation, manufacturing controlled-release matrices, and tableting.

The purpose of the PAT initiative is to move analytical laboratory functions close to the manufacturing process to improve manufacturing efficiency and product quality.

Downstream process design can increase facility output through improved overall process yield or higher batch capacity in mass and volume.

The overhead expense that comes along with each new enterprise application adds up quickly, and can be somewhat invisible to owners of the system.

Air filtration also needs a filter integrity test method to guarantee the sterility of critical parameters.

Minimum disruption and maximum gain result when adopting a distributed process control and data management system for a cell culture and fermentation lab.