
Amid debate about “fake news,” peer-review papers offer vital, objective insight.


Amid debate about “fake news,” peer-review papers offer vital, objective insight.

This article provides an overview on important aspects related to bracketing strategies in Japan.
The objective of this study was to assess the impact of manufacturing-scale, freeze-thaw conditions on aggregation and subvisible particle formation of a monoclonal antibody solution (mAb-A; IgG1) using a small-scale model.
This study aims at understanding the differences between porcine and bovine trypsin from both pancreatic and recombinant origins.

This report describes the first known attempt at quantifying the success of such processes in inducing nucleation on a 56–m2 freeze dryer operating at a load of 195,960 vials.

To investigate the best culture conditions, the authors used response surface methodology via Box-Behnken design.
An approach to stabilize PBS-based formulations could provide a simple physiological solution for use of proteins in research, preclinical, diagnostics, and clinical studies, as well as commercial biotherapeutic products.
This study offers a strategy for stabilization of biotherapeutics for long-term frozen storage in PBS-based formulations.
The study demonstrates a systematic approach to stabilize PBS-formulated mAbs against freeze-thaw degradation.

The authors present an overview of the types of RNA-based therapeutics in existence and their optimal methods of manufacture, purification, formulation, and delivery.
Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.
The authors provide application data to support the use of SEC beyond small-scale operations.
The authors describe the ways in which manufacturers can mitigate the risks related to the integrity of recombinant transgenes expressed in CHO cells.

The authors explore the use of precipitation using polyvinyl sulfonic acid and zinc chloride in place of capture chromatography to reduce the cost of goods in the insulin manufacturing process.

The authors evaluate the SoloVPE technique as a replacement for nitrogen-based protein determination.

This case study reviews how quality-by-design principles can be implemented in an intermediate chromatography purification step that uses cation-exchange chromatography.Abstract

Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.

The authors explore the use of statistical experimental design and multivariate analysis to develop a drug substance formulation matrix.
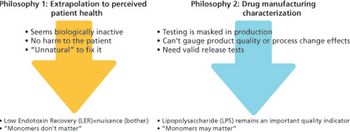
Low endotoxin recovery represents an opportunity to add value to the characterization of biologic drug products.
Differentiation and Characterization of Protein Aggregates and Oil Droplets in Therapeutic Products
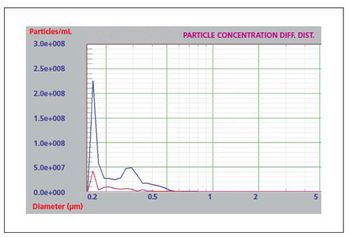
Liquid particle counters are ideal for protein aggregation studies.

The authors review efforts to limit polymer degradation without significantly impeding cell growth.

The use of orthogonal methods to SEC is discussed and examples are presented showing how analytical ultracentrifugation, AF4, and SEC compare in aggregate quantitation.

Fed-batch processes were scaled up from traditional bench-scale bioreactors to large-scale single-use systems.

Different approaches to prepare highly concentrated feed media for fed-batch Chinese hamster ovary cell culture are evaluated.