
The “next-generation” design for the pods will build on Pfizer’s existing modular prototype for oral solid-dose manufacturing.

The “next-generation” design for the pods will build on Pfizer’s existing modular prototype for oral solid-dose manufacturing.

BioSC Lab biochromatography system performs protein purification in batch and continuous modes.


The House Energy and Commerce Committee gave unanimous approval to the landmark 21st Century Cures Act reform bill on May 21, 2015.

Higher cell densities, greater demand for high-performance viral clearance, and desire for large-scale single-use technologies are driving development of filtration technologies.

Making the switch from batch to continuous manufacturing requires a thorough understanding of the process.

The upgrades will offer the opportunity for higher product yields and higher purity levels.

The author discusses HTST pasteurization and UHT sterilization.

Unique technology expands Entegris? fluid-sensing and control offering.

Rutgers engineers constructed a direct compaction line in collaboration with Janssen.
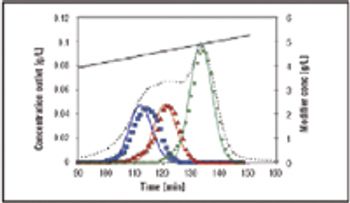
This article presents the multicolumn countercurrent solvent gradient purification (MCSGP) process, which uses three chromatographic columns, and incorporates the principle of countercurrent operation and the possibility of using solvent gradients. A MCSGP prototype has been built using commercial chromatographic equipment. The application of this prototype for purifying a MAb from a clarified cell culture supernatant using only a commercial, preparative cation exchange resin shows that the MCSGP process can result in purities and yields comparable to those of purification using Protein A.