
Lives are saved when time from vein to vein decreases.

Lives are saved when time from vein to vein decreases.

Sartorius’ acquisition of Polyplus is designed to strengthen its cell and gene therapy capabilities.

TreeFrog Therapeutics is leaping ahead in cell therapies through resources such as new technologies and investor partnerships.

The combined solutions are currently available on the market.

Key challenges posed to autologous and allogeneic treatments could be resolved by in-vivo CAR-T gene therapies.

The new guidance provides detailed recommendations to drug developers with a target of helping to ensure that drug developers provide adequate information to assess potency at each stage of a product’s life cycle.
Decreasing vein to vein time saves lives.

There is growing pressure for robust and economically scalable viral-vector manufacturing technologies.

A slew of late-stage clinical trials is expected to push new regenerative medicines onto the market in the next few years.

Improvement in viral vector yield has become integral to new cell and gene therapy product development.
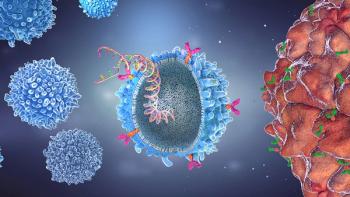
In vivo CAR-T gene therapies could overcome the challenges faced by autologous and allogeneic treatments.

CellVax Therapeutics has selected Theragent, a new CDMO, to manufacture clinical trial material for a new Phase II prostate cancer immunotherapy drug candidate.

The newly formed partnership between CSafe and BioLife will expand cold chain supply chain solutions for the cell and gene therapy market.


Biopharma focuses on streamlining biomanufacturing and supply chain issues to drive uptake of cell and gene therapies.

Document integrity is critical in the provision of raw materials for cell and gene therapy manufacturing.

For cell and gene therapies to reach their full potential, changes in manufacturing must be explored.

Forecyte Bio and Cytiva will team up to accelerate the development and manufacturing of cell and gene therapies.

The clinical trial research environment has evolved because of specific solutions designed to overcome uncertainty.

Pfizer and Touchlight have signed a patent license agreement for Pfizer to use Touchlight’s doggybone DNA (dbDNA) in the manufacture of mRNA vaccines, therapeutics, and gene therapies.
Finding specific solutions to overcome uncertainty has led to the evolution of a new clinical trial research environment.

Inceptor Bio and the University of Minnesota aim to build a novel iPSC platform to accelerate cell therapy drug development.

Precompetitive consortiums seek solutions to industry-wide challenges.

AGC Biologics is investing in viral vector suspension technology at its new Longmont, Colo., facility.

Through a collaboration, Avantor and Cytovance Biologics will accelerate plasmid optimization and sourcing services for viral vectors and mRNA-based vaccines and therapeutics.