
USP releases compendium of quality standards for compounded medicines.

USP releases compendium of quality standards for compounded medicines.

USP expresses its support for a consensus-based global approach to the naming of biologics.

The European Pharmacopoeia Commission re-evaluates its policy on the development of monographs for finished drug products.

USP releases new and revised standards for organic impurities in medicines for public comment.

USP opens expanded Shanghai facility to enhance quality standards for medicines and food ingredients.

The international “Fight the Fakes” campaign will raise awareness about the dangers of counterfeit medicines.

USP issues call for candidates for its 2015-2020 Council of Experts.

USP is developing and revising distribution standards in response to changes in the global supply chain.

By embracing efficiency and quality, biopharmaceutical organizations can work better and achieve better work.

USP appoints regulatory experts to elemental impurities implementation advisory group.

USP defers implementation date to work closely with ICH Q3D. USP will also form a new advisory group for implementation of the new general chapters on elemental impurities.

The U.S. Pharmacopeial Convention is offering free online access to public standards to help ensure the quality of the herbal ingredients used in medicinal products.

New Center for Pharmaceutical Advancement and Training increases number of experts and available tools in Sub-Saharan countries.

Two general chapters on elemental impurities? limits and procedures are to become official Feb. 1, 2013, with implementation proposed for May 1, 2014.

USP revises labeling requirements for Heparin.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.
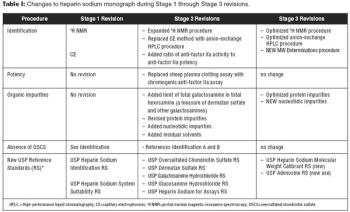
USP optimizes identification tests and impurities procedures.

USP Hosts Symposium on Science and Standards

Brazil's regulatory health authority, Anvisa, plans to establish quality requirements for locally produced pharmaceutical excipients, Anvisa told BioPharm International.

US Pharmacopeia promotes horizontal standards and a product-class approach for quality attributes.

US Pharmacopeia develops and improves its class approach for ensuring quality biopharmaceuticals.

USP is advancing efforts to develop a guidance for evaluating bioassays.

The US Pharmacopeia (Rockville, MD, USP, www.usp.org) recently announced that the implementation period for its USP–NF general notices statement requiring all manufacturers to conform to recently revised residual solvent standards in General Chapter <467> has been extended from July 1, 2007 to July 1, 2008.

The United States Pharmacopeia (USP, Rockville, MD, www.usp.org) and the UK's National Institute for Biological Standards and Control (NIBSC, Hertfordshire, UK, www.nibsc.ac.uk) are seeking participants in a study of analytical methods used by the industry to characterize and quantify oligosaccharides.