
The agency has assigned new EU member state rapporteurs and co-rapporteurs to medicines previously assigned to the UK’s Medicines and Healthcare products Regulatory Agency.

The agency has assigned new EU member state rapporteurs and co-rapporteurs to medicines previously assigned to the UK’s Medicines and Healthcare products Regulatory Agency.
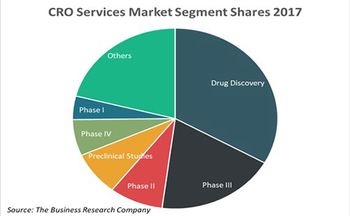
A new study by the Business Research Company reveals prominent contract research organization outsourcing trends.

David Weingarten, PhD, and Mark Feldstein, PhD, will present ways to optimize pharma patent protection to avoid generic competition and increase return on investment at CPhI North America 2018.

Serialization technology and implementation expert Rick Seibert will present an insight briefing on recent serialization challenges at CPhI North America in Philadelphia, PA.

Pharma event announces plans to alternate locations between Indonesia and Thailand to open new market opportunities.

National Institutes of Health researchers use genomics to show that squamous cell carcinomas differ from other cancers, which could advance treatments for head and neck and other cancers.

CPhI North America 2018 is hosting a forum for female pharmaceutical professionals to network, collaborate, and share their perspectives at this year��’s conference.

Shashank Upadhye, Esq., managing partner at Amin Talati Upadhye, will discuss intellectual property/patent issues for active pharmaceutical ingredients and abbreviated new drug applications at CPhI North America in Philadelphia, PA, on Tuesday, April 24, 2018.

On Tuesday, April 24, 2018, Evan Boswell, senior principal scientist at Pfizer CentreOne Contract Manufacturing, Pfizer CentreOne will give a presentation on scaling up the manufacturing process of active pharmaceutical ingredients at CPhI North America in Philadelphia, PA.

The European Pharmacopoeia Commission added 19 new monographs and three new chapters and revised 51 monographs and 15 chapters.

The agency’s plan outlines its approach to implementing medical product programs and the use of financial resources.

A new scientific publication examines analytical processes for the emerging legal cannabis industry.

The agency has release a report providing an overview of steps taken to enhance benefit-risk assessment in the review of drugs.

The company’s Krios G3i cryo-electron microscope is a finalist in the 2018 Edison Awards.

Congress passed a $1.3-trillion omnibus budget bill March 22, 2018 that increases federal support for biomedical research and health programs for fiscal year 2018.

Major countries will be ranked based on the potential for biopharma market growth, innovation, and competitiveness.

The agency’s Committee for Medicinal Products for Human Use recommended six drugs for approval at its March 2018 meeting.

The draft guidance addresses the agency’s policy on evaluating bulk drug substances in drug compounding.

AbbVie and the International Myeloma Foundation will partner to study the role of a genetic mutation in outcomes of patients with multiple myeloma.

The agency’s Clinical Data Summary Pilot program will post redacted Clinical Study Reports in order to help stakeholders understand why FDA approved a new drug application.

The agency sent a warning letter to Malladi Drugs & Pharmaceuticals Limited after an inspection found CGMP violations that included the presence of vermin.

ISPE announced BioMarin Pharmaceutical, Shire, Vetter, and Wyeth Pharmaceuticals as FOYA Category winners.

The agency published two new guidance documents detailing postmarketing safety reporting requirements for combination products.

Emerson will provide Ireland’s National Institute of Bioprocessing Research and Training with technologies to help the institute prepare students for the transition to manufacturing digitization in the biopharmaceutical industry.

The agency announced proposed research studies on how healthcare providers and patients understand drug promotional materials.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

The FDA commissioner outlined the agency's initiatives to reward innovation and biosimilars development.

GlobalData reports the need to shift away from egg-based manufacturing of vaccines in light of influenza-related deaths.

The company is recalling three lots of Hydromorphone HCl Injection USP CII 10 mg/mL, 1 mL in 2 mL Single Dose Vials because of possibly empty or cracked vials.

The company is recalling Methylprednisolone Sodium Succinate for Injection, USP, 40mg, 125mg, and 1g manufactured by Gland Pharma Ltd and distributed by Sagent due to out-of-specification impurity results.