
The National Institute of Allergy and Infectious Diseases began a first-in-human trial of an experimental live, attenuated Zika virus vaccine.

The National Institute of Allergy and Infectious Diseases began a first-in-human trial of an experimental live, attenuated Zika virus vaccine.

FDA approved the first generic version of EpiPen and EpiPen Jr (epinephrine) auto-injector.

FDA grants support US research in continuous manufacturing monitoring and control techniques for bio/pharmaceutical manufacturing at Rutgers, MIT, and Georgia Tech.

FDA sent a warning letter to Apotex Research Private Limited after investigators found current good manufacturing practice violations.

FDA published a resource guide to promote responsible opioid prescribing in the treatment of animals.

The agency issued a warning letter to Canadian API manufacturer, Les Produits Chimiques B.G.R, citing cGMP violations at its API facility in Pointe-Claire, Quebec.
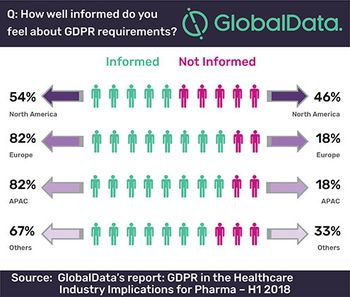
Only 54% of North Americans feel informed about the requirements of the general data protection regulation (GDPR), according to a report by GlobalData.

The Alliance for Regenerative Medicine’s (ARM) report highlights major trends and metrics from the 2018 second quarter in the regenerative medicines sector.

Ebola vaccinations by the World Health Organization began in North Kivu, Democratic Republic of the Congo, one week after the country’s latest outbreak.

The European Medicines Agency (EMA) will temporarily scale back activities as it copes with “significant staff loss” and prepares for the next phase in its continuity plan.

Avalon and Weill will co-develop bio-production and standardization procedures for CAR-T therapy.

Boehringer Ingelheim joins Oxford BioMedica, UK Cystic Fibrosis Gene Therapy Consortium, and Imperial Innovations to form a partnership for developing a new gene therapy to treat cystic fibrosis.

AuroMedics Pharma issued a voluntary, nationwide recall of two lots of piperacillin and tazobactam for injection, USP 3.375 g due to the presence of particulates identified as glass and silicone material.

Researchers at the University of Pennsylvania found that diabetes drugs called thiazolidinediones can promote the metabolism of glutamine to help control disease-causing inflammation.

The recommended drugs include two orphan medicines and three biosimilars.

The National Science Foundation grant will be used to commercialize a synthetic biology platform for cancer drug development.

Commissioner Gottlieb is reorganizing FDA in the hopes of streamlining policymaking.

Rising pressure to do more to control drug prices is prompting Trump administration officials to explore where more flexible import policies may help ensure access to safe, effective, and more affordable medicines.

The agency announced two research collaborations on bulk lists, updates to categories of bulk drug substances, and issued a warning about a bulk drug substance used in compounding.

FDA and ICH seek comment on new exposure levels for cadmium in drug products.

Inspectors found quality issues at bB BioChem Laboratories Inc, a California-based manufacturer of over-the-counter drug products.

In launching FDA’s Biosimilar Action Plan, Gottlieb takes innovator companies to task for delaying competitive biosimilar products.

FDA Commissioner, Scott Gottlieb, announced formation of a new, drug shortages task force and efforts to advance long-term solutions to prevent shortages.

Teva Pharmaceuticals, Major Pharmaceuticals, and Solco Healthcare are voluntarily recalling their products containing valsartan because of the presence of N-nitrosodimethylamine.

CPhI Korea to feature zone for finished dosage formulation drug products.

The agency is releasing six new draft guidances to provide a regulatory framework for handling gene therapies.

Annual awards program recognizes innovation in bio/pharmaceutical drug development and manufacturing.

Progressive Care’s DischargeRX program works with hospitals and patients to help improve patient medication adherence and minimize medication-related hospital readmissions.

Findings from a survey show that some companies have not yet taken the necessary steps to ensure the continuity of medicine supply in the European Union after the United Kingdom’s departure.

Report predicts PAT, NIRS, continuous bioprocessing, and a ‘technological arms race’ could improve biopharma manufacturing efficiencies.