For cellular materials, new ultra scale-down devices inform large-scale downstream processing techniques.

For cellular materials, new ultra scale-down devices inform large-scale downstream processing techniques.

Chromatography modeling can enhance bioprocessing efficiencies.
FTIR can successfully measure key characteristics of therapeutic proteins in a single step.

The Cannabis Analyzer for Potency from Shimadzu Scientific Instruments (SSI) is a high-performance liquid chromatograph (HPLC) specifically for quantitative determination of cannabinoid content.

The Thinky PR-1 Nanoparticle Dispersion Machine from Intertronics is a desktop unit that can disperse carbon nanotubes (CNT), graphene, and other 2D nanomaterials within a closed container.

The Quantum Pump from Watson Marlow Fluid Technology Group is a peristaltic pump with patented ReNu single-use (SU) cartridge technology.

The X500B QTOF System is the latest solution in SCIEX’s X-Series Quadrupole Time of Flight (QTOF) mass spectrometry (MS) platform.
The author discusses the impact of prefilled syringe product contact materials and the filling and stoppering process on protein aggregates.
OrlaSURF technology can be used for the development of target-binding assays to monitor the binding of an ADC to its antigen.

As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the value of internal audits and how the information gained can be applied.
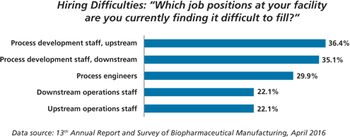
New study shows China biopharma companies face staffing shortages.

Industry fears limited benefits as FDA readies voluntary data tracking program.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
Including next-gen antibodies in pharma pipelines is considered essential for future success.
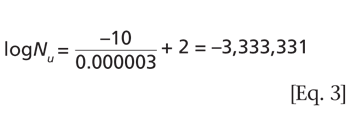
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

Click the title above to open the BioPharm International April 2017 issue in an interactive PDF format.