
Proper selection and installation optimizes fluid system performance.

Proper selection and installation optimizes fluid system performance.

The Wilden Quattroflow QF10k size pump from PSG, a Dover company, has been designed to fill the gap between the existing QF4400/5050 and QF20k pump sizes.
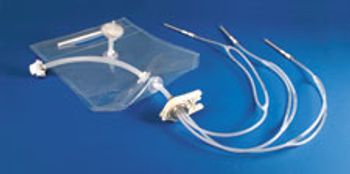
Sterile-molded filling assemblies from AdvantaPure are suited for single-use vial and syringe filling.

Pharmaceutical companies and contract manufacturing organizations report lack of readiness for the November 2018 US Drug Supply Chain Security Act serialization deadline.

A review of 2017 advancements from the Open-SCS working group with Charlie Gifford, group technical director.

New technological advancements seek to improve pharmaceutical process monitoring and control.

Open standards based on GAMP and GS1 will soon be released and more companies are moving beyond basic compliance.

Scientific advances and renewed investment may infuse biopharma for growth.

BioPharm International will mark 30 years of biopharma industry progress and challenges in 2018.

Gene therapies highlight FDA new drug approvals in 2017.
Advances in TFF and single-use systems help advance UF/DF on the continuous processing path.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

Policy makers look to boost generic drugs, curb opioid abuse, and maintain incentives for innovation.
Recent investments show expansion activity in cell culture facilities.

The industry will see an impact from financing, M&As, advanced therapies, generic drugs, and the retail market in 2018.

Enterprise-wide processes, procedures, and systems are the keys to data integrity and peace of mind, according to Siegfried Schmitt, PhD, principal consultant at PAREXEL.

Click the title above to open the BioPharm International January 2018 issue in an interactive PDF format.