
Suitable for bioprocessing, solubilization, and stabilization applications, the new histidine products allow for the control of endotoxin and trace metal impurities reflected with elemental impurity data in parts per billion on their specifications.

Suitable for bioprocessing, solubilization, and stabilization applications, the new histidine products allow for the control of endotoxin and trace metal impurities reflected with elemental impurity data in parts per billion on their specifications.
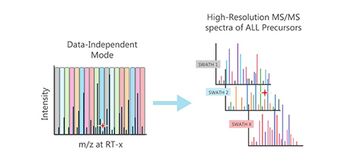
This method is expected to help bring gene therapies to market faster, safer, and cheaper.

The agency is hoping to restart performing on-site domestic inspections during the week of July 20, 2020 depending on factors such as the status of COVID-19 in the state of inspection and local rules and guidelines.

The agreement will accelerate production of SiO2’s plastic vials for vaccines and therapeutics for Operation Warp Speed.

Stevanato Group’s Vision Robot Unit uses AI-based machine learning capabilities for particle and cosmetic inspection of biopharmaceutical drug products.

Through the acquisition, the companies plan to build a global science-based organization that uses product innovation to enhance ABITEC’s scientific and commercial interests.

The new SDMS automatically gathers and protects all instrument and other laboratory data as it is generated for enhanced lab management, quality, and security.

VAR2 Pharmaceuticals has been selected for its development of a drug-conjugated malaria protein that potentially selectively binds to most human tumor types.

BARDA and DOD have awarded a $450-million contract to Regeneron Pharmaceuticals to manufacture and supply an investigational double antibody cocktail in development for treating COVID-19.

The acquisition will give the CDMO additional clinical filling options in Europe, which is expected to come online in 2021.

The company will manufacture a conjugated vaccine candidate being developed to prevent the disease melloidosis.

SGS has announced the receipt of approval from Belgium’s Federal Agency for Medicines and Health Products (FAMHP) to begin a new clinical trial of a potential treatment for patients with COVID-19 related respiratory failure.

The European Commission has approved Lynparza (olaparib) for use in patients with germline BRCA-mutated (gBRCAm) metastatic pancreatic cancer within the European Union.

GlaxoSmithKline (GSK) and Medicago have announced a collaboration for the development and evaluation of a COVID-19 vaccine candidate.

The agreement will fund the late-stage clinical development, a Phase III clinical trial, large-scale manufacturing, and the delivery of 100 million doses of Novavax’s COVID-19 vaccine candidate, NVX‑CoV2373.

The new site will offer initial design and development, low-volume manufacturing, and scalable high-quality production of wearable drug delivery devices.

The European Directorate for the Quality of Medicines and Healthcare (EDQM) has published a new general chapter (2.6.32) in the European Pharmacopoeia (Ph. Eur.) supplement 10.3.

Emergent is entering into a five-year agreement with Janssen Pharmaceuticals for the large-scale drug substance manufacturing of Johnson & Johnson’s investigational COVID-19 vaccine, Ad26.COV2-S.

The agreement will combine Rentschler’s experience in drug substance manufacturing and Vetter’s expertise in aseptic fill/finish and secondary packaging.

The new facility in Grand Rapids, MI, is part of the CDMO’s aggressive expansion plan.

Intravacc will license CimCure’s iBoost technology, and the companies will jointly develop a COVID-19 vaccine candidate.

While there currently is no molecular therapy targeted against the PAUFgene, Prestige believes its monoclonal antibody has the potential to benefit patients affected by PAUF-positive pancreatic cancer.

As a result of disruptions caused by COVID-19, CPhI Worldwide 2020 in-person event will not be taking place in Milan, Italy, but instead will be a unique digital experience to connect the global pharma industry.

Phesgo is a fixed-dose-combination subcutaneous injection of Perjeta (pertuzumab) and Herceptin (trastuzumab) for treating HER2-positive breast cancer.

The complete response letter was issued for a biologics license application for Abicipar pegol, an investigational treatment for wet age-related macular degeneration, based on FDA’s determination of an unfavorable benefit–risk ratio.

Aji Bio-Pharma launched Ajility, a flexible platform for manufacturing vaccines and therapies.

The guidance document provides recommendations regarding data needed for the manufacturing, development, and approval of a COVID-19 vaccine.

The company now offers its CONFIDENCE virus clearance services to support validation of viral clearance processes.

The expansion included adding new softgel encapsulation lines at its Strathroy, Canada and Sorocaba, Brazil sites for Catalent’s Vegicaps plant-based capsule and its CosmoPod twist-off capsule technologies.

The site can now support customized product and bioprocess development and custom cell and gene therapy reagent manufacturing.