
MedImmune and 3M Drug Delivery Systems collaborate to develop toll-like receptor (TLR) agonists for immuno-oncology.

MedImmune and 3M Drug Delivery Systems collaborate to develop toll-like receptor (TLR) agonists for immuno-oncology.

US Compounding, Inc. issues voluntary recall of all sterile products.

Six grants from the NIH will help identify variants in the genome’s regulatory regions that affect disease risk, using new computational approaches.

Novo Nordisk will build a local manufacturing plant for FlexPen prefilled insulin delivery devices in Iran.

Tomas Salmonson is re-elected as Chair of the Committee for Medicinal Products for Human Use.

FDA issues warning letter to Jaychem Industries, Auckland, New Zealand for violating CGMP regulations.

The agency cited Pan Drugs Limited with improper cleaning of facilities and equipment.

The protective suit was recently honored by Fast Company’s 2015 Innovation by Design Awards.

Novovax’s investigational vaccine targeting respiratory syncytial virus (RSV) demonstrated clinical effectiveness in animal models.

Nexvet Biopharma, a veterinary biologics developer, secured a dedicated, cGMP biologics manufacturing facility in Tullamore, Ireland, and plans to invest in disposable technology.

Daiichi Sankyo signed an exclusive licensing agreement with clinical-stage biopharmaceutical company Translational Sciences to develop and commercialize its novel thrombus (blood clot) dissolving agent, TS23.

President Obama will nominate Rob Califf, current deputy commissioner for medical products and tobacco, as FDA commissioner.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

FDA announced the recall, citing deficiencies in Medistat’s aseptic processing areas and in its environmental monitoring procedures.

Wilden’s Saniflo Hygienic Series pumps use a new, energy-efficient air distribution technology.
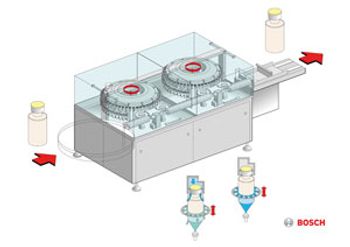
The RAN 3080 exterior washing machine from Bosch cleans filled and closed vials, ampuls, and cartridges using a high-pressure process and special transportation system.

Aragen Bioscience has licensed ProteoNic Biotechnology’s 2G UNic recombinant protein production technology, which increases manufacturing efficiency and reduces cost of goods for recombinant biologicals.

FDA warns that compounded or repackaged drugs stored in certain syringes made by Becton-Dickinson may lose potency because of an interaction with the rubber stopper.

FDA publishes the final Q3D Elemental Impurities guidance.

The agency has set up a workshop on how to demonstrate the benefits of orphan drugs over existing treatments.

The agency releases guidance on the nonclinical evaluation of endocrine-related drug toxicity.

The agency releases guidance on good review practice for formal dispute resolution in regards to appeals.

Reuters reports that Novo Nordisk will invest 500 million Danish Krone (approximately $75 million US dollars) into a new 19,000 m2 warehouse in Hillerød, Denmark. The warehouse will handle all inbound raw materials for Novo Nordisk's production in Denmark and will have a capacity of around 17,000 pallets of product, according to the news outlet.

The platform, developed by Vectron Biosolutions, will be used for BI’s in-house pipeline and for its outsourcing clients.

The collaborative effort will be focused on fully humanized antibodies.

Under the terms of the agreement, Sartorius Stedim Biotech will manufacture membrane adsorber technologies for GE, which will be marketed as part of GE’s ReadyToProcess product offerings.

UPS joins the Global Health Supply Chain Technical Assistance program to help secure the drug supply chain.

The agency started the full operation of its medical literature monitoring on Sept. 1, 2015.

Adents’ Pharma Suite serialization software features track-and-trace capabilities.

Pharma scientists announced for AAPS executive council and section leadership positions.