
EMA Executive Director Guido Rasi outlines his plan for the agency, including a focus on R&D.

EMA Executive Director Guido Rasi outlines his plan for the agency, including a focus on R&D.

Changes in China’s Food and Drug Administration (cFDA) drug development and commercialization policies make it easier for multinationals and CMOs to manufacture in China for in-country use, reports CMO and consultant PaizaBio.

Assembly Biosciences reported a successful clinical study validating the use of the Gemicel technology platform for the oral delivery of biologic medications.

The United States Pharmacopeial Convention announced the recipients of the 2015–2016 Global Fellowship Awards.

FDA approves Kanuma, the first treatment for patients with lysosomal acid lipase.

On Dec. 8, 2015, Sandoz, the generic pharmaceutical’s division of Novartis, announced that the European Medicines Agency (EMA) has accepted their marketing authorization application (MAA) for a biosimilar to Enbrel (etanercept), a tumor necrosis factor alpha (TNF-alpha) inhibitor.

Amgen submitted a market authorization application to EMA for ABP 501, a biosimilar candidate to Humira.

FDA released an interim response to AbbVie’s citizen petition on the labeling of biosimilars.

TxCell signs strategic agreement with MaSTherCell for European manufacturing of its cell therapy products.

FDA sets a July 2016 deadline for the final version of the rule on labeling changes for approved drugs and biologics.

Scientists at Rice University have developed a method to control the infectivity of viruses and gene delivery to the nuclei of target cells.

Adaptimmune Therapeutics and Universal Cells enter into collaboration and license agreement to develop universal allogeneic T-cell therapies.

Paragon Bioservices entered into a contract with the International Aids Vaccine Initiative for the process and analytical development and cGMP manufacturing of an HIV vaccine candidate.

The agency publishes draft guidance on best practices for communication between FDA and IND sponsors during drug development.

Seqirus, CSL Limited’s influenza vaccine business, announced the opening of their corporate headquarters in the United Kingdom.

Biogen, Genentech, Johnson & Johnson, Novartis, and Patheon publicize their support for action on climate change.

Amgen announced the submission of a BLA with the FDA for ABP 501.

Vetter receives AEO-F certificate from the European Union for global movement of goods.

Paras Biopharmaceuticals and Novozymes will collaborate on the creation of an improved osteoporosis treatment.

The agency promotes safer use of drugs and prevention of medication errors through a new webpage and practice guide.

The portal is an interactive tool that pulls together the innovation landscape, company base, and manufacturing sites for the entire medicines sector in the UK.

Under the terms of the agreement, Xellia will acquire substantial parts of the Ben Venue site, including four sterile injectable manufacturing plants, which are not currently operational.

Chugai Pharmaceuticals, a research-based company headquartered in Tokyo, opens new facility in Berkley Heights, NJ.

The proposed merger of Pfizer and Allergan will create a new top drug maker and cut Pfizer’s tax bill with a headquarters move to Ireland.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.

Expansions at Catalent’s Kansas City, MO, and Madison, WI facilities made in response to industry demand.
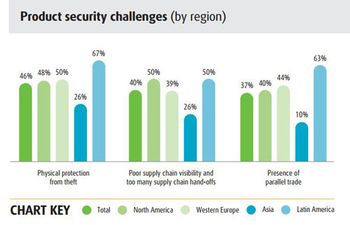
The 2015 UPS supply chain survey suggests that pharma companies need to improve cost control and planning for unexpected events.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Thermo Fisher Scientific announced the opening of their new GMP clinical services facility in Singapore.

Charles River Laboratories announces the acquisition of Oncotest GmbH.