Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.

Biopharma employees in different market segments note subtle differences in job satisfaction.

Biopharma and contract providers must tread carefully amid changing market dynamics.

A CPhI report suggests that the pharma industry should be careful not to outsource to lower labor-cost countries rather than investing in manufacturing technology and innovation.

A CPhI report predicts how two potential mega free-trade deals, one among Pacific-Rim countries and the other between the US and EU, could affect pharmaceutical companies.

In the development of biopharmaceuticals and pharmaceuticals, the line is blurring.

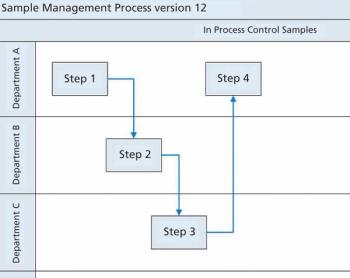
Ensuring data integrity involves effort on an individual and global basis.
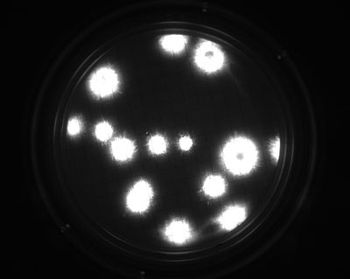
The rapid microbiological growth-based method represents an alternative for the quantification of contaminants in filterable products.

*This article is an opinion piece and does not necessarily represent the views of BioPharm International.

FDA notes progress in drug development, but cites scientific and funding roadblocks.

As biopharma enjoys success, it cannot ignore pressing patient access questions.

Objective, peer-reviewed papers and technical articles can help advance biopharmaceutical development.

FDA approves a biosimilar and loses a commissioner in March.

Sessions address cell therapies, serialization, contract services, and more.

Initiatives to speed drug development must pass Congress and special interest groups.

New designations lead to faster drug approvals, but there is more work to be done.

Ebola Virus Disease (EVD) is a severe, often fatal disease that is transmitted human-to-human through bodily fluids.

AAPS supports graduate-level programs impacted by cutbacks in funding and resources.

This article is the third and final in a series designed to offer leaders and managers at all levels in the industry a road map to excellence in human performance and human error prevention.

FDA draft guidances seek to maintain accurate drug information in new media.

Achieving excellence in human performance is an achievable system of cultivating and sustaining exceptional levels of performance.

Protecting intellectual property rights is vital to biopharmaceutical innovation.

Changes are needed to maintain US biopharma innovation leadership.

More media options open publishing opportunities for bioprocessing experts and authors.

Conference programming from PDA and BioPharm International expand educational opportunities at Interphex 2014.