
With some FDA inspections on hold, will the US drug supply maintain its quality standards?

With some FDA inspections on hold, will the US drug supply maintain its quality standards?

Monographs are developed based on the submission of information and materials from a company having regulatory approval for the product, and this submission feeds into the pharmacopoeia revision process.

This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

This article returns to the topic of complying with pharmacopoeial requirements with a case study at the intersection of monograph development and compliance.
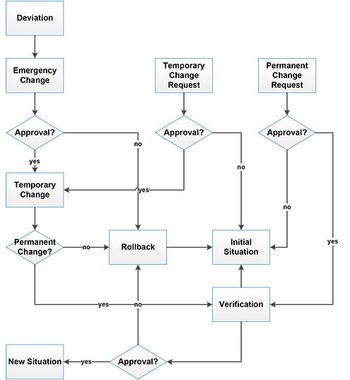
No matter why change may be needed, it is important to comply with all the relevant regulatory requirements, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

ERS Genomics revealed that the European Patent Office (EPO) has rejected arguments filed in opposition to patent EP2800811, which is directed to the single-guide CRISPR/Cas9 gene editing system and covers uses in cellular and non-cellular settings.

ICH will be taking industry comments under consideration when it revises its Q9 guideline in order to clarify QRM requirements, says Susan J. Schniepp, executive vice-president of post-approval pharma and distinguished fellow, Regulatory Compliance Associates.

The agency celebrates the efforts it has made in creating a system for the evaluation and supervision of medicines throughout the European Union.

As the facility becomes fully operational, the company believes the potential risk of a shortage of the product due to increasing demand will be significantly reduced.

The agency’s joint Big Data Task Force and the Heads of Medicines Agencies proposed actions for the use of big data to support innovation and public health.

The European Medicines Agency and its European partners have launched a pilot program for cooperation in the inspection of facilities that manufacture sterile drug products.

The revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world are described.

This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An effective surveillance program for monitoring the activities of pharmacopoeias around the world requires processes, people, and tools from across a company.

An understanding of global and national pharmacopoeias is crucial to understanding change processes and access to different markets.
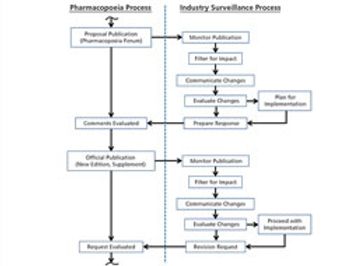
The process used to monitor and participate in pharmacopoeial changes is described.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.

Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

Galderma, a Swiss-based dermatology company that the United States Food and Drug Administration (FDA) has granted breakthrough therapy designation to its investigational therapy, nemolizumab.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.

CHMP has recommended that Ervebo (rVSVΔG-ZEBOV-GP), a vaccine for active immunization against Ebola, be granted conditional marketing authorization in the EU.

In this series of articles, the authors provide an understanding about the need for pharmacopoeia compliance and practical guidance to assist those who perform this work.

In this series of articles, the authors provide an understanding about the need for pharmacopoeia compliance and practical guidance to assist those who perform this work.

In this series of articles, the authors provide an understanding about the need for pharmacopoeia compliance and practical guidance to assist those who perform this work.