
The UK Pharma Industry has emphasised the need for the government to avoid a 'no-deal' Brexit in response to the prime minister delaying the deal vote.

The UK Pharma Industry has emphasised the need for the government to avoid a 'no-deal' Brexit in response to the prime minister delaying the deal vote.

Shire has announced that the European Commission has granted marketing authorisation for Takhzyro (lanadelumab) subcutaneous injection.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.

The positive opinions included the first oral-only tablet for the treatment of human African trypanosomiasis.

The agency provided an update on its relocation plans and assured that core activities are continuing uninterrupted.

The new good pharmacovigilance practice chapter IV on specific considerations for the pediatric population offers a holistic view of pediatric pharmacovigilance and provides guidance on how to address the specific needs and challenges of safety monitoring of medicines used in children.

Permission from the United Kingdom's Supreme Court has been granted to the UK BioIndustry Association, allowing it to intervene in a dosage regimen patent case.

Building up relevant expertise in-house will make writing spec sheets for software easier, according to Siegfried Schmitt, principal consultant at PAREXEL.

Takhzyro (lanadelumab) is the first monoclonal antibody therapy approved for the prevention of recurrent attacks of hereditary angioedema (HAE) in patients aged 12 years and older.

The recommended drugs include one biosimilar, two orphan medicines, and three extensions of therapeutic indication.

The contract development and manufacturing organization released its first serialized products to Europe from its facilities in Lisbon, Portugal and Stockholm, Sweden.

The Medicines and Healthcare products Regulatory Agency (MHRA) has opened a consultation to seek views on how legislations and procedures of the agency may need to be modified should there be a ‘no-deal’ Brexit scenario.

Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates, takes a look at the regulations around data integrity and how they relate to the concept of quality culture.

As Europe and the United Kingdom are facing the ever-expanding shadow of Brexit, a keynote session taking place at CPhI Worldwide in Madrid will look to assess the wider implications of the UK’s exit from the European Union on the Pharma sector.

The European Medicines Agency revised the number of centrally authorized medicines for which there are concerns over Brexit-related supply disruptions.

The European Medicines Agency’s detection of a second nitrosamine in a sartan API is driving a deeper dive into tetrazole chemistry; root-cause investigations will now include not only valsartan and losartan, but candesartan, irbesartan, and olmesartan.

The agreement now includes 15 European Union (EU) member states.

FDA is revising its inspection process and seeks harmonization of standards for US and foreign regulatory oversight to ensure the safety of medicines.

Detailed process descriptions and robust documentation aid in compliance as well as training, says Siegfried Schmitt, principal consultant at PAREXEL.

The European Commission (EC) has approved GlaxoSmithKline’s (GSK) Nucala (mepolizumab) as an add-on treatment for severe refractory eosinophilic asthma in pediatric patients six to 17 years old.

The European Commission (EC) has approved Novartis’ chimeric antigen receptor T cell (CAR-T) cell therapy Kymriah for the treatment of B-cell acute lymphoblastic leukemia and relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy.

Guidances for regulatory changes, batch testing, and reporting address situations resulting from “no-deal” Brexit scenario.

As a contingency against border delays resulting from a “no-deal” Brexit, the Department of Health and Social Care (DHSC) directs pharma companies to stock extra medicines.
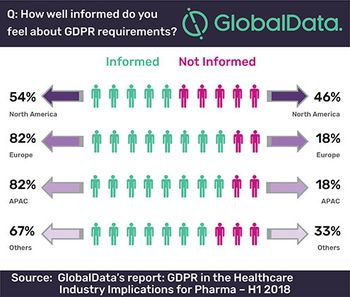
Only 54% of North Americans feel informed about the requirements of the general data protection regulation (GDPR), according to a report by GlobalData.

The European Medicines Agency (EMA) will temporarily scale back activities as it copes with “significant staff loss” and prepares for the next phase in its continuity plan.