
The European Commission and EMA have created an action plan to address challenges identified by stakeholders involved in developing advanced therapies.

The European Commission and EMA have created an action plan to address challenges identified by stakeholders involved in developing advanced therapies.

This approval marks the second gene therapy to be approved by FDA and the first to be approved for certain types of non-Hodgkin lymphoma.

The vaccine is a non-live, recombinant subunit vaccine that combines an antigen and an adjuvant system to trigger a targeted and long-lasting immune response to the shingle-causing virus.

Stelara (ustekinumab), already approved for treating adults, is now also approved for treating adolescents with moderate to severe plaque psoriasis.

The committee has voted unanimously to approve Spark Therapeutics’ gene therapy candidate, Luxturna (voretigene neparvovec), for treating a genetically inherited blindness.

Amgen is seeking approval for an additional indication in glucocorticoid-induced osteoporosis for its blockbuster osteoporosis therapeutic, Prolia.

AstraZeneca is seeking approval for its anti-cancer monoclonal antibody, Imfinzi, for treating non-small cell lung cancer in the European Union.

The approval marks the first biosimilar approved in the United States for treating cancers.

FDA issued a Refusal to File letter to Acorda Therapeutics, citing insufficiently complete information in the company’s new drug application for its investigational Parkinson’s disease drug.

The withdrawal has been attributed to issues at the biotech company’s manufacturing facility.

FDA urges manufacturers to seek fast approval of “high-need” generics and targeted therapies.

The agency is offering patients with life-threatening diseases a how-to tool on accessing investigational drugs.

The agency announced a plan to eliminate its existing orphan designation request backlog.

Approval of breakthrough therapies requires expedited quality assessment.

CBER is moving forward with the development and approval of regenerative medicine advanced therapies.

Sandoz, a Novartis division, announced that the Committee for Medicinal Products for Human Use (CHMP) has adopted positive opinions recommending the approval of its biosimilars rituximab and etanercept in Europe, for the same indications as the respective reference medicines.

The agency approved Renflexis, a biosimilar to Janssen’s blockbuster rheumatoid arthritis treatment.

Pharmaceutical Technology spoke with CPhI North America presenter Jonathan Helfgott to discuss navigating GDUFA and helpful tips for submitting successful ANDAs.

The mAb is the first approved treatment that targets the progressive form of the disease.

Will new generic drugs bring the cost of medicines down in the way policy makers hope?

In a FDAVoice blog post, CBER Director Peter Marks discusses the new designation for cell therapies that treat life-threatening diseases.
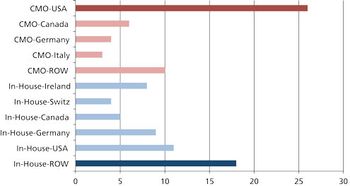
Moving global manufacturing operations may be more complicated than it appears.

In 2016, FDA approved 630 ANDAs and tentatively approved 183 ANDAs, the highest number to date, according to the report.

FDA granted inotuzumab ozogamicin priority review and accepted its BLA for filing.

The regulatory agency rejected the medication, citing various issues related to device use.